- Articaine HCl
-

- $0.00 / 1kg
-
2025-11-22
- CAS:27262-47-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 600kg
- Levobupivacaine
-

- $0.00 / 1kg
-
2025-11-04
- CAS:27262-47-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1000kg
- Levobupivacaine
-
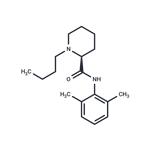
- $40.00 / 5mg
-
2025-11-02
- CAS:27262-47-1
- Min. Order:
- Purity: 99.72%
- Supply Ability: 10g
|
| | Levobupivacaine Basic information |
| | Levobupivacaine Chemical Properties |
| Melting point | 136-137°C | | alpha | D25 -80.9° (c = 5 in methanol) | | Boiling point | 430.65°C (rough estimate) | | density | 1.0238 (rough estimate) | | refractive index | 1.5700 (estimate) | | storage temp. | Refrigerator | | solubility | DMSO:58.0(Max Conc. mg/mL);201.09(Max Conc. mM)
Ethanol:58.0(Max Conc. mg/mL);201.09(Max Conc. mM) | | pka | 14.85±0.70(Predicted) | | form | Solid | | color | White to Off-White |
| | Levobupivacaine Usage And Synthesis |
| Chemical Properties | White Solid | | Originator | Chirocaine,Abbott Laboratories | | Uses | Local anaesthetic used for epidural and intrathecal anaesthesia. | | Definition | ChEBI: Levobupivacaine is the (S)-(-)-enantiomer of bupivacaine. It has a role as a local anaesthetic, an adrenergic antagonist, an amphiphile, an EC 3.1.1.8 (cholinesterase) inhibitor and an EC 3.6.3.8 (Ca(2+)-transporting ATPase) inhibitor. It is a conjugate base of a levobupivacaine(1+). It is an enantiomer of a dextrobupivacaine. | | Manufacturing Process | Synthesis of L-pipecolic acid 2,6-xylidide (Patent US 4,695,576)
130 g of pipecolic acid and 158.6 g of Laevo (+)-tartaric acid are dissolved
under stirring in 2 L 95% ethyl alcohol and 125 ml water at 80°C. The
solution is allowed to cool to room temperature and after two days the
crystallized D-pipecolic-tartrate is separated. The L-pipecolic-tartrate remains
in solution. The filtrate is evaporated and dissolved in 5% acetic acid. Finally
the solution is treated with Amberlite IR 45* in an ion exchanger. The eluate
thus obtained is evaporated and the resulting crystalline residue is dried with
potassium hydroxide in vacuo. The product obtained consists of L-pipecolic
acid [α]D24 = -26.2°(C = 5, H2O).
4 g of phosphorus pentachloride was added to a suspension of 4 g of L�pipecolic acid hydrochloride in 40 ml acetylchloride. The initial reaction is
effected at a temperature of about 35°C under stirring for 2 hours. The
chlorination is completed by adding during a time period of about 10 minutes
an additional two grams of phosphorus pentachloride and stirring over a
further period of 4 hours while maintaining the suspension at a temperature
of about 35°C. The resulting L-pipecolic acid chloride hydrochloride is filtered
and washed with toluene and acetone. The crystalline residue is then dried in
vacuo, m.p. 155°C.
A mixture of 2.7 ml 2,6-dimethylaniline, 4 ml acetone, and 4 ml N�methylpyrrolidone is gradually added under stirring for 2 hours at 70°C to a
suspension of 4 g of L-pipecolic acid chloride hydrochloride. This yields a
crystalline product, which is filtered, washed with acetone and dried. This
crystalline product is then dissolved in water and the base is precipitated by
the addition of ammonia. The base is then extracted by the use of toluene and
is recovered by evaporation. The base is recrystallized from a mixture of
hexane and ethanol to yield L-pipecolic acid 2,6-xylidide. The melting point of
this compound is 129-130°C.
Preparation of L-N-n-butylpipecilic acid 2,6-xylidide may de carried out by
analogy with the preparation of L-N-n-propylpipecolic acid 2,6-xylidide (Patent
US 5,777,124).
n-Butylbromide and potassium carbonate are added to a solution of L-pipecolic
acid 2,6-xylidide dissolved in isopropyl alcohol. Thereafter, 5 ml of water is
added to the mixture and the reaction is carried out for 4 hours at 72°C.
To complete the reaction, a further 0.8 ml n-butylbromide are added under
continuous stirring and heating for 4 hours. The residue is treated with a
mixture of 250 ml toluene and an equal amount of water at 50°C. The toluene
layer is separated and washed three times with 100 ml warm water (40°C). A
175 ml portion of the toluene is removed by evaporation and the remainder is
stored at +5°C for 6 hours to achieve crude crystalline L-N-n-butylpipecilic
acid 2,6-xylidide. The crystalline product is separated by filtration, washed
with some cooled toluene and dried at 70°C. Recrystallization may be carried
from toluene. This product is dissolved in 100 ml ethanol and neutralized with
concentrated hydrochloric acid. Ethanol is removed by evaporation and the
hydrochloride product obtained is vacuum dried. Finally the latter is
recrystallized from isopropyl alcohol. | | Therapeutic Function | Local anesthetic | | General Description | Levobupivacaine is the pure “S” enantiomer of bupivacaineand in vivo and in vitro studies confirm that it does notundergo metabolic inversion to R(+) bupivacaine. The pKaof the tertiary nitrogen is 8.09, the same as bupivacaine’s pKa. Levobupivacaine is available in solution for epiduraladministration, peripheral nerve block administration, andinfiltration anesthesia. Clinical trials have shown thatlevobupivacaine and bupivacaine have similar anestheticeffects. Levobupivacaine has lower CNS and cardiotoxicitythan bupivacaine although unintended intravenousinjection when performing nerve blocks may result intoxicity. Racemic bupivacaine is metabolized extensivelywith no unchanged drug found in the urine or feces. Liverenzymes including the CYP3A4 and CYP1A2 isoforms areresponsible for N-dealkylation and 3-hydroxylation oflevobupivacaine followed by glucuronidation or sulfation. | | Pharmacology | This is the laevo (S–) enantiomer of bupivacaine, with similar properties to
the racemic mixture, though it has slightly higher protein binding and
clearance and hence a lower potential for cardiac and CNS toxicity. In
practice, several other factors contribute to local anaesthetic toxicity, and the recommended maximum doses remain the same. Its
formulation is expressed as percentage weight per unit volume of free base;
racemic bupivacaine is expressed as percentage weight per unit volume of
hydrochloride salt. Levobupivacaine therefore contains 13% more active
molecules for a given dose. |
| | Levobupivacaine Preparation Products And Raw materials |
|