- Bupivacaine
-

- $50.00 / 10G
-
2024-09-23
- CAS:2180-92-9
- Min. Order: 10G
- Purity: 98%
- Supply Ability: 2000KG
- Bupivacaine
-

- $0.00 / 1KG
-
2024-09-09
- CAS:2180-92-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500
- Bupivacaine
-

- $0.00 / 25kg
-
2024-08-14
- CAS:2180-92-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
Related articles - What is Bupivacaine?
- Bupivacaine is the most potent and toxic of all amide anesthetics. It is four times more potent than lidocaine, mepivacaine, a....
- Sep 24,2020
|
| | Bupivacaine Basic information |
| Product Name: | Bupivacaine | | Synonyms: | (.+/-.)-1-Butyl-2',6'-pipecoloxylidide;1-Butyl-2-(2,6-xylycarbamoyl)piperidine;1-Butyl-2',6'-pipecoloxylidide;1-butyl-2’,6’-pipecoloxylidide;1-butyl-n-(2,6-dimethylphenyl)-2-piperidinecarboxamid;Bupivacaine Base & HCL;Bupivacaine anhydrous;Bupivacaine (USP) | | CAS: | 2180-92-9 | | MF: | C18H28N2O | | MW: | 288.43 | | EINECS: | 218-553-3 | | Product Categories: | research chemical;Active Pharmaceutical Ingredients;API | | Mol File: | 2180-92-9.mol | 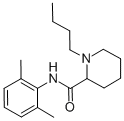 |
| | Bupivacaine Chemical Properties |
| Melting point | 107.5-108° | | Boiling point | 430.65°C (rough estimate) | | density | 1.0238 (rough estimate) | | refractive index | 1.5700 (estimate) | | pka | 8.09; also reported as 8.17(at 25℃) | | Water Solubility | 101.5mg/L(25 ºC) | | InChI | InChI=1S/C18H28N2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21) | | InChIKey | LEBVLXFERQHONN-UHFFFAOYSA-N | | SMILES | C1(CCCCN1CCCC)C(=O)NC1C(C)=CC=CC=1C | | CAS DataBase Reference | 2180-92-9(CAS DataBase Reference) | | NIST Chemistry Reference | 2-Piperidinecarboxamide, 1-butyl-n-(2,6-dimethylphenyl)-(2180-92-9) | | EPA Substance Registry System | 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)- (2180-92-9) |
| | Bupivacaine Usage And Synthesis |
| Originator | Carbostesin,Astra,W. Germany,1967 | | Uses | Anesthetic (local). | | Uses | Like lidocaine and mepivacaine, bupivacaine is used in infiltration, spinal, and epidural anesthesia in blocking nerve transmission. Its most distinctive property is its long-lasting action.
It is used for surgical intervention in urology and in lower thoracic surgery from 3 to 5 h in
length, and in abdominal surgery lasting from 45 to 60 min. It is used to block the trifacial
nerve, the sacral and brachial plexuses, in resetting dislocations, in epidural anesthesia, and
during Cesarian sections. | | Definition | ChEBI: 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide is a piperidinecarboxamide obtained by formal condensation of the carboxy group of N-butylpipecolic acid with the amino group of 2,6-dimethylaniline. It is a piperidinecarboxamide, an aromatic amide and a tertiary amino compound. It is a conjugate base of a 1-butyl-2-[(2,6-dimethylphenyl)carbamoyl]piperidinium. | | Brand name | Marcaine (Hospira); Sensorcaine
(AstraZeneca). | | Therapeutic Function | Local anesthetic | | General Description | Bupivacaine was synthesized simultaneously with mepivacainein 1957 but was at first overlooked because of the increasedtoxicity compared with mepivacaine. When themethyl on the cyclic amine of mepivacaine is exchanged fora butyl group the lipophilicity, potency and the duration ofaction all increase. Literature reports of cardiovascular toxicity,including severe hypotension and bradycardia, areabundant in the literature.91 Bupivacaine is highly bound toplasma proteins (95%), and thus the free concentration mayremain low until all of the protein binding sites are occupied.After that point, the plasma levels of bupivacaine rise rapidlyand patients may progress to overt cardiac toxicity withoutever showing signs of CNS toxicity. The cardiotoxicity ofbupivacaine is a result of its affinity to cardiac tissues and itsability to depress electrical conduction and predispose theheart to reentry types of arrhythmias. The cardiotoxicity ofbupivacaine was found to be significantly more prominentwith the “R” isomer, or the racemic mixture, thus the “S”stereoisomer is now on the market as levobupivacaine. | | Mechanism of action | Bupivacaine is a local anaesthetic containing a chiral centre and adopts dextro and laevo forms. The enantiopure l form is less cardio- and neurotoxic and has an equivalent potency to the racemic mixture; therefore levobupivacaine is often preferred to reduce the potential for toxicity. Stereoselectivity describes the differences in response at a given receptor for the different enantiomers (such as the response discussed for S(+) ketamine). The opioid and NMDA receptors also exhibit stereoselectivity. | | Pharmacology | Bupivacaine is a chiral compound used clinically for 50 years, with a slower
onset, greater potency and longer duration of action than lidocaine. Initial
benefits of bupivacaine were sensory–motor separation and minimal
tachyphylaxis, unlike repeated doses of lidocaine. However, it has greater
potential for cardiac toxicity, related to its avid binding to and slow
dissociation from cardiac N a+ channels. Inadvertent intravenous
administration may result in systemic toxicity (see later), and it is
contraindicated for intravenous regional anaesthesia.
Bupivacaine is commonly used for epidural administration in obstetrics and
postoperative pain management. A hyperbaric preparation containing
80 mg ml-1 glucose is available for spinal anaesthesia. | | Side effects | Common side effects of bupivacaine include:
weakness, long-lasting numbness or tingling;
feeling restless or drowsy;
tremors;
headache, blurred vision;
fast or slow heartbeats;
breathing problems;
chills or shivering;
back pain;
nausea, vomiting. | | Synthesis | Bupivacaine, N-2,6-(dimethyl)1-butyl-2-piperidincarboxamide (2.2.7), is chemically similar to mepivacaine and only differs in the replacement of the N-methyl sub�stituent on the piperidine ring with an N-butyl substituent. There are also two suggested meth�ods of synthesis. The first comes from α-picolin-2,6-xylidide (2.2.4). The alkylation of the last with butyl bromide gives the corresponding pyridine salt (2.2.6). Finally, it is reduced by hydrogen using platinum oxide as a catalyst into a piperidine derivative—bupivacaine.
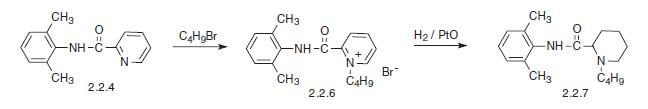
The other method results directly from the piperidine-2-carboxylic acid chloride, which is reacted with 2,6-dimethylaniline. The resulting amide (2.2.8) is further alkylated with butyl bromide to bupivacaine [17–19].
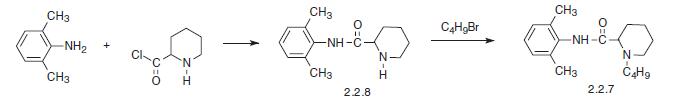
|
| | Bupivacaine Preparation Products And Raw materials |
|