- KANAMYCIN
-
- $0.00 / 25g
-
2023-11-01
- CAS:8063-07-8
- Min. Order: 1g
- Purity: 95%,97%,99%
- Supply Ability: 100kg
- KANAMYCIN USP/EP/BP
-
- $1.10 / 1g
-
2021-06-23
- CAS:8063-07-8
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | KANAMYCIN Basic information |
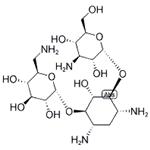
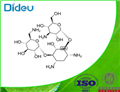
| Product Name: | KANAMYCIN | | Synonyms: | KANAMYCIN A;KANAMYCIN BASE;O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1->6)-O-[6-amino-6-deoxy-alpha-D-glucopyranosyl-(1->4)]-2-deoxy-D-streptamine;C00304;KANAMYCIN;Kanamycin
DISCONTINUED, UNDEFINED STRUCTURE, offer alternates K137523 or K137495 | | CAS: | 8063-07-8 | | MF: | C18H36N4O11 | | MW: | 484.5 | | EINECS: | 232-512-7 | | Product Categories: | | | Mol File: | 8063-07-8.mol | 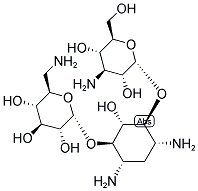 |
| | KANAMYCIN Chemical Properties |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | form | liquid | | Stability: | Stability Incompatible with strong oxidizing agents. |
| Hazard Codes | T | | Risk Statements | 61 | | Safety Statements | 36/37/39-45-53 | | WGK Germany | 2 | | HS Code | 29419000 |
| | KANAMYCIN Usage And Synthesis |
| Description | Kanamycin A belongs to the family on aminoglycoside antibiotics which consist of two or more aminosugars linked by glycosidic bonds to an aminocyclitol ring. It was isolated in Japan from Streptomyces kanamyceticus. Clinically, kanamycin is used as a sulfate. Currently, kanamycin is only rarely used; its main place is for the treatment of tuberculosis caused by multidrug-resistant Myobacterium tuberculosis strains . | | Chemical Properties | solid | | Originator | Kantrex,Bristol,US,1958 | | Indications | Kanamycin A is similar to streptomycin and neomycines and has a broad spectrum of antimi�crobial action. It is active with respect to most Gram-positive as well as Gram-negative
microorganisms (staphylococci, gastric bacilli, rabbit fever, Fridlender’s bacillus, proteus,
shigella, salmonella).
It is used for treating sepsis, meningitis, osteomyelitis, periotonitis, pneumonia,
pyelonephritis, pyelocystitis, infected wounds, and post-operational purulent complica�tions caused by microorganisms sensitive to the drug. Synonyms of this drug are karmycin,
kamaxin, resistomycin, and many others. | | Definition | ChEBI: Kanamycin A is a member of kanamycins. It has a role as a bacterial metabolite. It is a conjugate base of a kanamycin A(4+). | | Manufacturing Process | As described in US Patent 2,931,798, Streptomyces kanamyceticus (K2-J) was
first cultured in shake flasks in the following media: (a) 0.75% meat extract,
0.75% peptone, 0.3% NaCl, with 1.0% of starch, dextrin, maltose, glucose,
lactose, sucrose or glycerol; or (b) 2.0% soybean meal, 0.05% KCl, 0.05%
MgSO4 · 7H2O, 0.5% NaCl, 0.2% NaNO3, with 1.0% of starch, dextrin,
maltose, glucose, lactose, sucrose or glycerol. The initial pH of all media was
adjusted to 7.0. After 24 to 48 hours shaking in some cases the pH decreased
to about 6.0 to 6.8, but from 72 to 120 hours the pH rose and became 7.5 to
8.6. The production of kanamycin was apparent after 48 hours and, depending
on the media; the maximum production was found after 72 to 120 hours.
The yield was highest with starch or dextrin, intermediate and about the same
with sucrose, glucose, maltose and lactose and poorest with glycerol.
Kanamycin was produced by media containing soybean meal, peanut meal,
cottonseed meal, corn steep liquor, peptone, yeast extract or meat extract,
with or without sodium nitrate. Commercially available soybean meal was
recognized to be one of the best nitrogen sources. The addition of corn steep
liquor, peptone, yeast extract or nitrate to the soybean meal promoted the
production of kanamycin.
The brownish white kanamycin (5 g) was dissolved in 50 ml of 60% aqueous
methanol, insoluble material was removed and to the filtrate 40 ml of 60%
aqueous methanol containing 2,000 mg of ammonium sulfate was added, and
the precipitated kanamycin sulfate was collected, washed with 50 ml of 80%
aqueous methanol, and dried. Thus, 4.5 g of kanamycin sulfate was obtained
as a light brownish powder. | | Therapeutic Function | Antibacterial | | Biological Activity | Kanamycin is considerably broader in spectrum than streptomycin, kanamycin is more effective against gram-negative bacilli (other than Pseudomonas) and also is effective to a degree against Staph. aureus. However, it is ineffective against streptococci and pneumococci. The availability of penicillinase-resistant penicillins and cephalosporins essentially obsoleted kanamycin as the primary drug in the treatment of staphylococcal infections. Kanamycin has been essentially replaced by gentamicin and other aminoglycosides which are less ototoxic (adverse to hearing), and which also have a wider range of antibacterial activity. | | Mechanism of action | Kanamycin and other aminoglycoside antibiotics interfere with bacterial protein synthesis. The drug binds to a particular protein or proteins of the 30S subunit of bacterial ribosomes. This results in a misreading (or miscoding) of mRNA codons. Consequently, wrong amino acids are incorporated into growing peptide chains and nonsense bacterial proteins are formed. This effect alone may not be lethal to bacteria, yet kanamycin and other aminoglycosides are rapidly bactericidal. Numerous hypotheses have been put forward over the years to explain this. The bactericidal property may be related to the very tight binding of aminoglycosides to the ribsomes, which is essentially irreversible. The most likely explanation seems to be that kanamycin also leads to the production of abnormal membrane proteins of the bacterial cell, which cause alterations in membrane permeability, and this plays an essential role in the bactericidal action. | | Synthesis | Kanamycin, O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→6)-O-[6-deoxy-6-
amino-α-D-glucopyranosyl-(1→4)]–2-deoxy-D-streptamine (3.4.6), is isolated from a
culture liquid of the actinomycete S. kanamyceticus, which produces three antibiotics—
kanamycines A, B, and C. | | Drug interactions | All aminoglycosides are partially inactivated by high concentrations of any of the penicillins. In vitro, the penicillins inactivate kanamycin to about the same degree as gentamicin and tobramycin, but this occurs less readily with amikacin. Studies with gentamicin and amikacin have shown that heparin reversibly inhibits aminoglyco�side activity in a dose-dependent way. This may also apply to kanamycin. Specimens for kanamycin measurements should not be obtained in heparinized tubes. Kanamycin excretion is not affected by probenecid. |
| | KANAMYCIN Preparation Products And Raw materials |
|