| | OXAZEPAM Basic information |
| Product Name: | OXAZEPAM | | Synonyms: | 2h-1,4-benzodiazepin-2-one,7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-[qr];7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepin-2-one[qr];7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepine-2-one;7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepine-2-one[qr];7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-4-benzodiazepin-2-one;7-Chloro-3-hydroxy-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one;7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one[qr];7-Chloro-3-hydroxy-5-phenyl-1,3-dihydro-benzo[e][1,4]diazepin-2-one | | CAS: | 604-75-1 | | MF: | C15H11ClN2O2 | | MW: | 286.71 | | EINECS: | 210-076-9 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;API | | Mol File: | 604-75-1.mol | 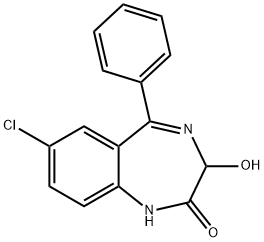 |
| | OXAZEPAM Chemical Properties |
| Melting point | 205-206° | | Boiling point | 506.5±50.0 °C(Predicted) | | density | 1.3052 (rough estimate) | | refractive index | 1.5200 (estimate) | | Fp | 11 °C | | storage temp. | −20°C | | solubility | Practically insoluble in water, slightly soluble in ethanol (96 per cent). | | pka | pKa 1.6/11.6(5% MeOH in H2O,t =20,I=0.15) (Uncertain) | | Water Solubility | 20mg/L(22 ºC) | | CAS DataBase Reference | 604-75-1(CAS DataBase Reference) | | IARC | 2B (Vol. Sup 7, 66) 1996 | | EPA Substance Registry System | Oxazepam (604-75-1) |
| | OXAZEPAM Usage And Synthesis |
| Chemical Properties | Off-White Solid | | Originator | Serax,Wyeth,US,1965 | | Uses | Anxiolytic; muscle relaxant (skeletal); anticonvulsant; ligand for the GABAA receptor benzodiazepine modulatory site. Controlled substance (depressant). | | Definition | ChEBI: A 1,4-benzodiazepinone that is 1,3-dihydro-2H-1,4-benzodiazepin-2-one substituted by a chloro group at position 7, a hydroxy group at position 3 and phenyl group at position 5. | | Manufacturing Process | (A) Suspend 10 g of 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2one 4-oxide in 150 ml of acetic anhydride and warm on a steam bath with stirring until all the solid has dissolved. Cool and filter off crystalline, analytically pure 3-acetoxy-7-chloro-1,3-dihydro-5-phenyl-2H-1,4benzodiazepin-2-one, melting point 242°C to 243°C.
(B) Add to a suspension of 3.4 g of 3-acetoxy-7-chloro-1,3-dihydro-5-phenyl2H-1,4-benzodiazepin-2-one in 80 ml of alcohol.6 ml of 4 N sodium hydroxide. Allow to stand after complete solution takes place to precipitate a solid. Redissolve the solid by the addition of 80 ml of water. Acidify the solution with acetic acid to give white crystals. Recrystallize from ethanol to obtain 7chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one, melting point 203°C to 204°C. | | Brand name | Serax (Alpharma); Zaxopam (Quantum Pharmics). | | Therapeutic Function | Tranquilizer | | General Description | Odorless creamy-white to pale-yellow powder or white crystalline solid. Bitter taste. pH (2% aqueous suspension) 4.8-7. | | General Description | Oxazepam, 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazpin-2-one (Serax), isan active metabolite of both chlordiazepoxide and diazepamand can be considered a prototype for the 3-hydroxy benzo-diazepines. It is much more polar than diazepam. Oxazepam is rapidlyinactivated to glucuronidated metabolites that are excretedin the urine. Thus, the half-life of oxazepam is about 4 to 8hours, and it is marketed as a short-acting anxiolytic. As aresult, its cumulative effects with chronic therapy are muchless than with long-acting benzodiazepine such as chlordiazepoxideand diazepam. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | OXAZEPAM is stable in light and is non hygroscopic. OXAZEPAM is stable in neutral solution. OXAZEPAM is hydrolyzed by acids and bases. | | Fire Hazard | Flash point data for OXAZEPAM are not available; however, OXAZEPAM is probably combustible. | | Pharmacokinetics | The half-life of oxazepam is approximately 4 to 8 hours, and cumulative effects with chronic therapy
are much less than with long-acting benzodiazepines, such as chlordiazepoxide and diazepam. Lorazepam is the
2′-chloro derivative of oxazepam and has a similarly short half-life (2–6 hours) and pharmacological activity. | | Clinical Use | Oxazepam | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: metabolism possibly increased by
rifampicin.
Antipsychotics: enhanced sedative effects; risk of
serious adverse effects in combination with clozapine.
Antivirals: possibly increased concentration with
ritonavir.
Sodium oxybate: enhanced effects of sodium oxybate
- avoid.
Ulcer-healing drugs: metabolism inhibited by
cimetidine. | | Metabolism | Oxazepam is an active metabolite of both chlordiazepoxide and diazepam and is marketed separately, as a short�acting anxiolytic agent. Oxazepam is rapidly inactivated to glucuronidated metabolites that are excreted in the urine. |
| | OXAZEPAM Preparation Products And Raw materials |
|