| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Amfenac
CAS:51579-82-9
Purity:> 95% Remarks:https://www.bocsci.com/product/amfenac-cas-51579-82-9-466443.html
|
| Company Name: |
SynAsst Chemical.
|
| Tel: |
021-60343070 |
| Email: |
|
| Products Intro: |
Product Name:2-(2-AMino-3-benzoylphenyl)acetic acid
CAS:51579-82-9
Purity:96%Min Package:10g 100g 500g
|
|
| | amfenac Basic information |
| Product Name: | amfenac | | Synonyms: | 2-Amino-3-benzoylbenzeneacetic acid;SOYCMDCMZDHQFP-UHFFFAOYSA-N;Nepafenac Impurity 5(Amfenac);Benzeneacetic acid, 2-amino-3-benzoyl-;Amfenac D5Q: What is
Amfenac D5 Q: What is the CAS Number of
Amfenac D5 Q: What is the storage condition of
Amfenac D5 Q: What are the applications of
Amfenac D5 | | CAS: | 51579-82-9 | | MF: | C15H13NO3 | | MW: | 255.27 | | EINECS: | | | Product Categories: | | | Mol File: | 51579-82-9.mol | 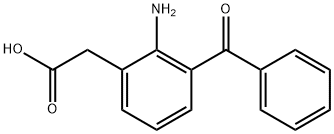 |
| | amfenac Chemical Properties |
| Melting point | 121-123° (dec) | | Boiling point | 398.5°C (rough estimate) | | density | 1.1654 (rough estimate) | | refractive index | 1.5500 (estimate) |
| Toxicity | LD50 in mice, rats (mg/kg): 615, 311 orally (Sancilio) |
| | amfenac Usage And Synthesis |
| Originator | Amfenac sodium,Yungjin Pharmacetical
Co. Ltd. | | Uses | Anti-inflammatory. | | Definition | ChEBI: An oxo monocarboxylic acid that is benzophenone in which one of the phenyl groups is substituted by an amino group and a carboxymethyl group at position 2 and 3, respectively. The corresponding carboxamide, nepafenac, is a prodrug of amfenac and is used fo
the treatment of pain and inflammation following cataract surgery. | | Manufacturing Process | 7-Benzoylindolin-2-one:
Method A. A mixture of 2.5 g (0.0077 mole) of ethyl 2-acetamido-3-
benzoylphenylacetate in 50 ml of 3 N hydrochloric acid was refluxed for one
hour. The reaction mixture was filtered and the filtrate was poured into a
mixture of ice and water. The precipitate was collected and recrystallized from
acetone; yield of 7-benzoylindolin-2-one 1 g (55%); melting point 154°C.
Method B. A solution of 1.3 g (0.0044 mole) of 2-acetamido-3-
benzoylphenylacetic acid in 15 ml of 3 N hydrochloric acid and 15 ml of acetic
acid was refluxed for three hours. The cooled solution was poured into ice
water and the 7-benzoylindolin-2-one which precipitated was collected and
dried.
2-Amino-3-benzoylphenylacetic acid:
A mixture of 1.0 g (0.004 mole) of 7-benzoylindolin-2-one was added to 30
ml of 3 N sodium hydroxide and the basic solution was refluxed for 45 min
under nitrogen. The mixture was filtered and the filtrate was neutralized with
glacial acetic acid. The precipitate was filtered off, washed with water and
dried. The 2-amino-3-benzoylbenzeneacetic acid melted at 122°C (dec.). The
yield was 0.8 g (72%). | | Therapeutic Function | Antiinflammatory |
| | amfenac Preparation Products And Raw materials |
|