- Amfenac sodium/Fenazox
-

- $0.00 / 1Kg/Bag
-
2025-11-29
- CAS:61618-27-7
- Min. Order: 1KG
- Purity: 98.5%min
- Supply Ability: 500kg
- Amfenac Sodium Hydrate
-
- $34.00 / 100mg
-
2025-10-27
- CAS:61618-27-7
- Min. Order:
- Purity: 99.67%
- Supply Ability: 10g
- Fenazox
-

- $15.00 / 1KG
-
2021-07-13
- CAS:61618-27-7
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Fenazox Basic information |
| | Fenazox Chemical Properties |
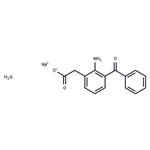
| Melting point | 242-244°C | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | Methanol (Slightly), Water (Slightly) | | form | Solid | | color | Yellow | | InChI | InChI=1S/C15H13NO3.Na.H2O.H/c16-14-11(9-13(17)18)7-4-8-12(14)15(19)10-5-2-1-3-6-10;;;/h1-8H,9,16H2,(H,17,18);;1H2; | | InChIKey | JFFPHRSIYAJPMA-UHFFFAOYSA-N | | SMILES | C(C1C=CC=CC=1)(C1C=CC=C(CC(=O)O)C=1N)=O.[NaH].O |
| | Fenazox Usage And Synthesis |
| Description | Amfenac sodium is a non-steroidal antiinflammatory agent structurally related to
ketoprofen,suprofen(10) and tiaprofenic acid.It is reported to be effective in the short term treatment of rheumatoidarthritis,osteoarthritis and pain associated with minor surgical procedures. | | Chemical Properties | Yellow Solid | | Originator | A.H. Robins (USA) | | Uses | Amfenac is an antibacterial agent | | Uses | Fenazox is used in the synthetic preparation of amides via reductive amidation of esters, which is used in the synthesis of bio-active molecules and natural products. | | Definition | ChEBI: A hydrate that is the monohydrate of the sodium salt of amfenac. | | Manufacturing Process | 7-Benzoylindolin-2-one:
Method A. A mixture of 2.5 g (0.0077 mole) of ethyl 2-acetamido-3-
benzoylphenylacetate in 50 ml of 3 N hydrochloric acid was refluxed for one
hour. The reaction mixture was filtered and the filtrate was poured into a
mixture of ice and water. The precipitate was collected and recrystallized from
acetone; yield of 7-benzoylindolin-2-one 1 g (55%); melting point 154°C.
Method B. A solution of 1.3 g (0.0044 mole) of 2-acetamido-3-
benzoylphenylacetic acid in 15 ml of 3 N hydrochloric acid and 15 ml of acetic
acid was refluxed for three hours. The cooled solution was poured into ice
water and the 7-benzoylindolin-2-one which precipitated was collected and
dried.
2-Amino-3-benzoylphenylacetic acid:
A mixture of 1.0 g (0.004 mole) of 7-benzoylindolin-2-one was added to 30
ml of 3 N sodium hydroxide and the basic solution was refluxed for 45 min
under nitrogen. The mixture was filtered and the filtrate was neutralized with
glacial acetic acid. The precipitate was filtered off, washed with water and
dried. The 2-amino-3-benzoylbenzeneacetic acid melted at 122°C (dec.). The
yield was 0.8 g (72%). | | Brand name | FENAZOX | | Therapeutic Function | Antiinflammatory | | General Description | Amfenac (Fenazox), its amide prodrug, nepafenac (Nevanac),and the related analog, bromofenac, are amphoteric becauseof the presence of an additional aromatic amine group.They are less likely to be absorbed into the general circulation.They are approved for use as topical ocular anti-inflammatoryagents for the treatment of postoperative ocular pain,inflammation, and posterior segment edema. The only observedside effects of these drugs are all related to tissuesaround the eye including abnormal ocular sensation, eye rednessand irritation, burning and stinging, and conjunctival orcornea edema. | | Synthesis | The reaction of 1-aminoindolin-
2-one with phenylacetone in presence of
acetic acid in refluxing ethanol gives 1-(α-
methylphenethylidieneimino)indolin-2-one,
which by reaction with refluxing ethanolic
hydrogen chloride affords ethyl α-(2-methyl-
3-phenylindol-7-yl)acetate. The ozonolysis of
this intermediate in acetic acid yields ethyl 2-
acetamido-3-benzoylphenyl acetate, which is
cyclized by refluxing with HCl in acetic acid
to give 7-benzoylindolin-2-one. Alternatively,
the hydrolysis of the ester ethyl α-(2-methyl-3-
phenylindol-7-yl)acetate with KOH in refluxing
water affords the corresponding acid, which can
be ozonolyzed as before yielding 2-acetamido-
3-benzoylphenylacetic acid. This acid can be
cyclized to 7-benzoyl-1,3-dihydro-indol-2-one
by refluxing with HCl in acetic acid as before
. |
| | Fenazox Preparation Products And Raw materials |
|