| Company Name: |
Tetranov Biopharm
|
| Tel: |
13526569071 |
| Email: |
sales@leadmedpharm.com |
| Products Intro: |
Product Name:2-(3-broMopyridin-2-yl)acetonitrile
CAS:122851-60-9
Purity:95% Package:1g;5g;25g;100g
|
| Company Name: |
Nanjing Chemlin Chemical Co., Ltd
|
| Tel: |
025-83697070 |
| Email: |
info@chemlin.com.cn |
| Products Intro: |
Product Name:3-Bromo-2-pyridineacetonitrile
CAS:122851-60-9
Purity:0.98 Package:5KG; 1KG
|
| Company Name: |
Synchem OHG
|
| Tel: |
+49 5662 408730 |
| Email: |
info@synchem.de |
| Products Intro: |
Product Name:(3-Bromopyridin-2-yl)acetonitrile
CAS:122851-60-9
|
|
| | 2-(3-bromopyridin-2-yl)acetonitrile Basic information |
| Product Name: | 2-(3-bromopyridin-2-yl)acetonitrile | | Synonyms: | (3-BROMOPYRIDIN-2-YL)ACETONITRILE;3-Bromopyridine-2-acetonitrile;3-BroMo-2-pyridineacetonitrile;2-(3-bromopyridin-2-yl)acetonitrile;2-Pyridineacetonitrile, 3-bromo-;2-(3-Bromo-2-pyridyl)acetonitrile | | CAS: | 122851-60-9 | | MF: | C7H5BrN2 | | MW: | 197.03 | | EINECS: | | | Product Categories: | | | Mol File: | 122851-60-9.mol | 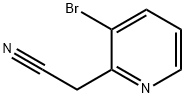 |
| | 2-(3-bromopyridin-2-yl)acetonitrile Chemical Properties |
| Melting point | 63-64 °C(Solv: ethyl ether (60-29-7)) | | Boiling point | 279.5±25.0 °C(Predicted) | | density | 1.575±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | 0.82±0.22(Predicted) | | Appearance | White to yellow Solid |
| | 2-(3-bromopyridin-2-yl)acetonitrile Usage And Synthesis |
| Synthesis | General procedure for the synthesis of 3-bromo-2-pyridineacetonitrile from 2,3-dibromopyridine and acetonitrile: A tetrahydrofuran (THF, 8 mL) solution of acetonitrile (87 mg, 2.13 mmol) was slowly added to a THF solution of n-butyllithium (n-BuLi, 1.78 mL, 4.26 mmol) at -78 °C and stirred for 1 hour at -78 °C. Subsequently, a THF (2 mL) solution of 2,3-dibromopyridine (500 mg, 2.13 mmol) was slowly added at the same temperature. The reaction mixture was continued to be stirred at -78 °C for 1.5 h and then slowly warmed up to room temperature. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride (NH4Cl) solution and the organic phase was extracted with ethyl acetate (EA, 100 mL x 3). The combined organic phases were washed with brine, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The residue was purified by preparative high performance liquid chromatography (HPLC) with the mobile phase of acetonitrile/water (containing 10 nM ammonium bicarbonate, NH4HCO3) to afford the target product 3-bromo-2-pyridineacetonitrile (110 mg, 22% yield) as a white solid. Liquid chromatography-mass spectrometry (LCMS) analysis showed [MH]+ = 197. | | References | [1] Synlett, 2000, # 10, p. 1488 - 1490
[2] Patent: WO2017/221092, 2017, A1. Location in patent: Paragraph 00161
[3] Patent: US2012/277247, 2012, A1. Location in patent: Page/Page column 12 |
| | 2-(3-bromopyridin-2-yl)acetonitrile Preparation Products And Raw materials |
|