| Company Name: |
Hu Bei Jiutian Bio-medical Technology CO.,Ltd |
| Tel: |
027-88013699 17354350817 |
| Email: |
Ryan@jiutian-bio.com |
| Products Intro: |
Product Name:1-Piperazinecarboxylicacid, 3-oxo-4-(phenylmethyl)-, 1,1-dimethylethyl ester
CAS:78551-60-7
Purity:0.99 Package:25kg,50kg,180kg,200kg,250kg,1000kg,as your needs Remarks:as your needs
|
|
|
|
|
| Company Name: |
Shanghai Chainpharm Bio-medical Technology Co., Ltd.
|
| Tel: |
+86-021-61727342 13611721451 |
| Email: |
chainpharm@163.com |
| Products Intro: |
Product Name:4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
CAS:78551-60-7
Purity:97 Package:1g/;10g/;100g/;250g/;500g/;1000g/;25k'g/
|
| Company Name: |
Suzhou JingTong Pharmaceutical Co., Ltd.
|
| Tel: |
13675155016 |
| Email: |
tongtf110@sina.com |
| Products Intro: |
Product Name:tert-Butyl 4-benzyl-3-oxopiperazine-1-carboxylate
CAS:78551-60-7
Purity:0.98 Package:50g?,1kg?,10kg
|
| Company Name: |
Aikon International Limited
|
| Tel: |
025-66113011 13155353615 |
| Email: |
qzhang@aikonchem.com |
| Products Intro: |
Product Name:tert-Butyl 4-benzyl-3-oxopiperazine-1-carboxylate
CAS:78551-60-7
Purity:95+% Package:1g;5g;10g
|
|
| | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER Basic information |
| Product Name: | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER | | Synonyms: | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER;tert-butyl 4-benzyl-3-oxopiperazine-1-carboxylate;1-BENZYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE;4-(t-Butoxycarbonyl)-1-(phenylmethyl)piperazinone;1-Piperazinecarboxylic acid, 3-oxo-4-(phenylmethyl)-, 1,1-dimethylethyl ester;Butyl-4-benzyl-3-oxopiperazine-1-carboxy late;1-Benzyl-4-Boc-piperazin-2-one | | CAS: | 78551-60-7 | | MF: | C16H22N2O3 | | MW: | 290.36 | | EINECS: | | | Product Categories: | | | Mol File: | 78551-60-7.mol | 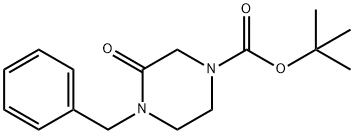 |
| | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER Chemical Properties |
| | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER Usage And Synthesis |
| Synthesis | Step 1: Synthesis of tert-butyl 4-benzyl-3-oxopiperazine-1-carboxylate
Sodium hydride (60% in mineral oil, 18.11 g, 452 mmol) was mixed with 35 mL of hexane, ground and dried under nitrogen protection and subsequently suspended in 1500 mL of tetrahydrofuran. To this suspension, 1-Boc-3-piperazinone (75.057 g, 200.4 mmol) was added in batches at 0 °C and the addition process lasted for 15 min. After 90 minutes of reaction, benzyl bromide (71.01 g, 415.1 mmol) was added and then the reaction mixture was gradually warmed up to room temperature and stirred for 18 hours. Upon completion of the reaction, the reaction was quenched with deionized water and extracted with ether. The combined organic layers were washed sequentially with water and brine and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product, which was purified by recrystallization from hexane to give tert-butyl 4-benzyl-3-oxopiperazine-1-carboxylate (83.5 g, 76% yield) as a white crystalline solid. | | References | [1] Patent: US2009/209553, 2009, A1. Location in patent: Page/Page column 14-15
[2] Tetrahedron Letters, 1996, vol. 37, # 41, p. 7339 - 7342
[3] Synthesis, 2005, # 3, p. 465 - 469
[4] Patent: US2004/259884, 2004, A1. Location in patent: Page 13
[5] Bioorganic and Medicinal Chemistry, 2008, vol. 16, # 3, p. 1431 - 1443 |
| | 4-BENZYL-3-OXOPIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER Preparation Products And Raw materials |
|