8-MERCAPTOADENOSINE manufacturers
- 8-MERCAPTOADENOSINE
-
- $0.00 / 1G
-
2025-09-23
- CAS:3001-45-4
- Min. Order: 1G
- Purity: 97%
- Supply Ability: 2000
|
| | 8-MERCAPTOADENOSINE Basic information |
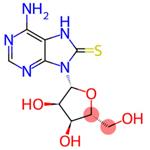
| Product Name: | 8-MERCAPTOADENOSINE | | Synonyms: | 8-MERCAPTOADENOSINE;7,8-Dihydro-8- thioxo- adenosine;8-Thioadenosine;6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-7H-purine-8(9H)-thione;Adenosine,7,8-dihydro-8-thioxo-;6-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-8,9-dihydro-7H-purine-8-thione | | CAS: | 3001-45-4 | | MF: | C10H13N5O4S | | MW: | 299.31 | | EINECS: | | | Product Categories: | | | Mol File: | 3001-45-4.mol | 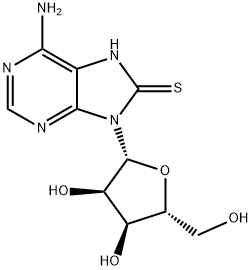 |
| | 8-MERCAPTOADENOSINE Chemical Properties |
| storage temp. | 2-8°C(protect from light) |
| | 8-MERCAPTOADENOSINE Usage And Synthesis |
| Uses | 8-Mercaptoadenosine is a nucleoside derivative that can be synthesized in DMF-aqueous solution at room temperature using NaSH via the corresponding 8-bromo compounds, and it is an intermediate in the synthesis of the substrate for RNA pyrophosphorylase[1]. | | Synthesis | The general procedure for the synthesis of 6-amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-3,4-diol of 6-amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-7H-purine-8(9H)-thione from (2R,3R,4S,5R)-2-(6-amino-8-bromo-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-2-yl) was as follows :
1. NaSH (0.8 g, 10 equiv) was added to a solution of 8-bromoadenine (0.5 g, 1.44 mmol) in DMF (7 mL).
2. heat the mixture to 100 °C and add a small amount of water to increase solubility.
3. Stir the reaction mixture at 100°C overnight.
4. the solvent is evaporated under high vacuum and the residue is co-evaporated with methanol several times until a solid is formed.
5. the residue is dissolved in water and neutralized with NaOH solution.
6. After freeze-drying, the product was purified by silica gel column chromatography (eluent: CHCl3:MeOH = 10:1).
7. The target compound was obtained as a light yellow powder (100% yield, melting point 169-170°C).
Product characterization data:
1H-NMR (CD3OD, 200 MHz): δ 8.09 (s, 1H, H-2), 6.65 (d, J = 7 Hz, 1H, H-1'), 5.01 (dd, J = 7, 5.5 Hz, 1H, H-2'), 4.39 (dd, J = 5.5, 2.5 Hz, 1H, H-3'), 4.13 (q, J = 2.5 Hz. 1H, H-4'), 3.87 (dd, J = 12.5, 2.5 Hz, 1H, H-5'), 3.71 (dd, J = 1.25, 3 Hz, 1H, H-5'').
13C-NMR (CD3OD, 300 MHz): δ 167.88 (C-6), 151.92 (C-2), 148.12 (C-4), 147.88 (C-8), 107.00 (C-5), 88.62 (C-1'), 85.59 (C-4'), 70.70 (C-2'), 70.62 (C-3') , 62.13 (C-5').
MS (CI/NH3): m/z 317 [M + NH4]+. | | References | [1] M Ikehara, et al. Studies of nucleosides and nucleotides. LVI. A versatile method for the systhesis of 8-mercaptoadenosine nucleotides. Chem Pharm Bull (Tokyo). 1973 Feb;21(2):444-5. DOI:10.1248/cpb.21.444 |
| | 8-MERCAPTOADENOSINE Preparation Products And Raw materials |
|