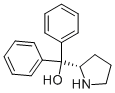
(S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol: properties, synthesis and safety
Nov 3,2023
General Description
(S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol is a chiral compound commonly used in the pharmaceutical industry as a chiral auxiliary, with reported analgesic, anti-inflammatory, and neuroprotective effects. Its synthesis involves a series of chemical reactions starting from benzaldehyde and pyrrolidine, with the resulting compound finding applications in various fields such as pharmaceutical chemistry, materials science, and agrochemical synthesis. Proper handling and safety precautions should be taken when working with (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol due to its potential hazards, including skin, eye, and respiratory irritation. Overall, the properties and applications of this compound make it an important component in the production of various medications that can improve human health and well-being.

Figure 1. (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol
Properties
(S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol is a chiral compound with the molecular formula C17H19NO and a molecular weight of 253.34 g/mol. It is a white to beige crystalline powder or crystals with a melting point range of 77-80°C (lit.), making it easily distinguishable from other compounds. It has an alpha value of -59 º (c=3, methanol 25 ºC) and a refractive index of -66.5° (C=3, CHCl3). Its solubility in chloroform is high. The predicted pKa value for this compound is 13.15±0.29. It is typically stored under an inert atmosphere and at room temperature to avoid any degradation. The compound is commonly used in the pharmaceutical industry as a chiral auxiliary, especially in the synthesis of asymmetric molecules. It has been reported to exhibit analgesic, anti-inflammatory, and neuroprotective effects that make it a useful component in drug development. The properties of (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol make it an important compound in both the academic and industrial research fields, facilitating the production of various medications that can improve human health and well-being. 1
Synthesis
The synthesis of (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol starts with the condensation reaction between benzaldehyde and pyrrolidine in the presence of a catalyst. This reaction forms the intermediates, α,α-Diphenyl-2-pyrrolidinemethanone. The next step involves the reduction of the ketone group in this intermediate using a reducing agent such as sodium borohydride or lithium aluminum hydride. This reduction yields (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol. The stereochemistry of the final product is determined by the chiral nature of the starting materials and the control of reaction conditions. The presence of a chiral catalyst or the use of enantiopure starting materials can lead to the formation of specifically one enantiomer, in this case, the (S)-enantiomer. (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol finds applications in various fields such as pharmaceutical chemistry, materials science, and agrochemical synthesis. It is commonly used as a resolving agent, chiral auxiliary, or ligand in asymmetric reactions. Its unique structure and chiral nature contribute to its versatility and importance in organic chemistry. In conclusion, the synthesis of (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol involves a series of chemical reactions starting from benzaldehyde and pyrrolidine. The resulting compound has significant applications in asymmetric catalysis and organic synthesis. 2
Safety
(S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol is a chemical compound that requires proper handling and safety precautions due to its potential hazards. It is important to understand and adhere to the recommended safety guidelines when working with this substance. (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol has been identified as a skin irritant, which means it can cause irritation, redness, or itching if it comes into contact with the skin. Therefore, it is crucial to wear appropriate personal protective equipment (PPE) such as gloves, goggles, and lab coats when handling (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol to minimize the risk of skin exposure. Furthermore, (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol can also cause serious eye irritation. In the event of accidental contact with the eyes, immediate flushing with water should be performed, and medical attention sought if symptoms persist. Additionally, (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol may cause respiratory irritation, emphasizing the importance of working in a well-ventilated area or using proper fume hoods to prevent the inhalation of vapors or dust particles. It is important to note that (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol should be stored in a cool, dry place away from incompatible materials and sources of ignition. Proper labeling and segregation of the chemical are necessary to avoid any potential risks. 3
Reference
1. PubChem. COMPOUND SUMMARY: (S)-(-)-alpha,alpha-Diphenyl-2-pyrrolidinemethanol. National Library of Medicine, 2005, CID: 2724899.
2. J.V. BK, Mariappan P. Convenient method for the synthesis of chiral α,α-diphenyl-2-pyrrolidinemethanol. Tetrahedron, 1993, 49(23): 5127-5132.
3. (S)-(-)-alpha,alpha-Diphenyl-2-pyrrolidinemethanol. European Chemicals Agency, EC / List no. 601-153-1.
- Related articles
- Related Qustion
- (S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol- Reaction / Application on synthetic works Nov 20, 2019
(S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol (DPPM) is a bifunctional organocatalyst, which could be used as a catalyst and organic synthesis reagent.
(S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol
112068-01-6You may like
(S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol manufacturers
- (S)-()-α,α-Diphenyl-2-pyrrolidinemethanol
-
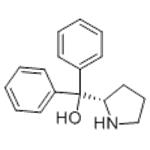
- 2025-12-11
- CAS:112068-01-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- diphenyl-[(2S)-pyrrolidin-2-yl]methanol
-
![112068-01-6 diphenyl-[(2S)-pyrrolidin-2-yl]methanol](/ProductImageEN2/2025-10/Small/aedfcfa1-de66-4059-9e0b-deb9f6b56239.jpg)
- $0.00 / 10kg
- 2025-10-24
- CAS:112068-01-6
- Min. Order: 100kg
- Purity: 99%min
- Supply Ability: 100 tons
- Dapoxetine Impurity 72
-
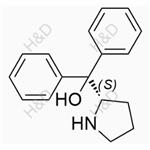
- $0.00 / 10mg
- 2025-09-25
- CAS:112068-01-6
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g