1,3,5-triglycidyl isocyanurate- Application and Toxicology
Feb 28,2020
Industrial Applications
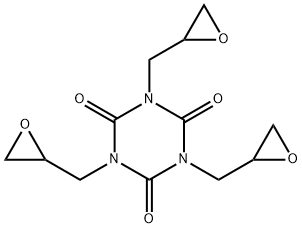
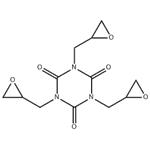
1,3,5-triglycidyl isocyanurate (TGIC) is a white powder or granular solid and is normally sold under the trade name ARALDITE PT810R or TEPIC G R. As a tri-functional epoxy monomer, TGIC is used in large scale as curing agent for powder coatings or for cross-linking elastomers. Rosin acids of natural origin may also be cured with TGIC for versatile applications. TGIC has also been used for pharmaceutical purposes, in membranes, for curing intelligent gels or liquid crystal elastomers. Especially, this curing agent has been widely used in dry powder coating for over 40 years. Industry claims are that the powders melt and chemically react during heat curing to form a stable polymeric matrix in which TGIC is chemically bound to the polymer, and cannot contaminate the environment (Powder Coating Institute)[1]
Atomic Structure
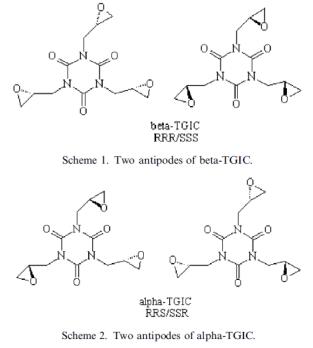
Triglycidyl isocyanurate has three chiral carbon atoms, the number of optical isomers should be eight (23 = 8). Due to its symmetrical structure, four antipodes may be distinguished, in two the configuration of the chiral carbon atoms is the same, i.e. RRR and SSS, and in the other two antipodes the configuration of one chiral carbon atom is different from that of the other two chiral carbon atoms, i.e. RRS and SSR. [3]
Health Toxicity
It has been known that TGIC is toxic by inhalation and if swallowed. It is a severe eye irritant, and a mild skin and nasal irritant, although it has the potential to cause skin sensitisation in people which can lead to severe skin rashes. Exposure to TGIC in powder paints may cause not only contact dermatitis, but also occupational asthma. Kidney damage has also been observed in rats repeatedly exposed to TGIC by oral dosing, and damage to the developing sperm cells has been observed in mice repeatedly exposed to TGIC orally and by inhalation. There is an evidence that exposure of males to TGIC produces genetic changes in the sperm which may lead to heritable effects in the offspring. There is cause for concern that these effects could occur in humans, so that TGIC has been classified in the EU as a Category 2 mutagen. Exposures to TGIC during manufacturing procedures have been identified as hazardous to workers. Therefore, TGIC-free mechanisms for the dry powder coating process are being investigated. [2]
References
1. Vargha V. Binary solid–liquid phase diagram of the two diastereomer racemates of triglycidyl isocyanurate (TGIC)[J]. European Polymer Journal, 2007, 43: 4762–4769.
2. Hou NY. Epoxy resin-based ultrafine dry powder coatings for implants[J]. J. Appl. Polym. Sci. 2016, DOI: 10.1002/APP.43960
Ma K. Simultaneously improving the flame retardancy and mechanical properties for polyamide 6/aluminum diethylphosphinate composites by incorporating of 1,3,5‐triglycidyl isocyanurate[J]. Polym Adv Technol. 2018, 29:1068–1077.
- Related articles
- Related Qustion
- Application, Mechanism of action, and Synthesis of 1,3,5-Triglycidyl isocyanurate Nov 11, 2021
Ethyl valerate (CAS No: 2451-62-9), also known as the green apple flavor is well known for its wide applications in the areas of food, pharmaceuticals and cosmetics industries.
- What is 1,3,5-Triglycidyl isocyanurate? Oct 11, 2021
1,3,5-Triglycidyl isocyanurate (Teroxirone) (CAS No: 2451-62-9), appears as a while crystalline solid, is a triazene triepoxide with anticancer and antineoplastic activity [1]. Teroxine alkylates and cross-links DNA, thereby inhibiting DNA
1H-Isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- is an important intermediate for the synthesis of rivaroxaban.....
Feb 27,2020Pharmaceutical intermediatesDeriving from seneciphylline, acetylseneciphylline N-oxide has a role as a Jacobaea metabolite. Structurally, it is an acetate ester, a macrocyclic lactone, an olefinic compound and an organic heterotricyclic compound.....
Feb 28,2020Inorganic chemistry1,3,5-Triglycidyl isocyanurate
2451-62-9You may like
1,3,5-Triglycidyl isocyanurate manufacturers
- 1,3,5-Triglycidyl isocyanurate
-
- 2025-12-14
- CAS:2451-62-9
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 1,3,5-Triglycidyl isocyanurate
-

- $10.00 / 1KG
- 2025-12-11
- CAS:2451-62-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Triglycidyl isocyanurate
-

- $29.00 / 5g
- 2025-12-05
- CAS:2451-62-9
- Min. Order:
- Purity: 99.01%
- Supply Ability: 10g