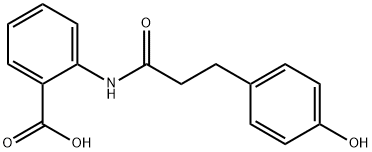
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid: activities and applications
Aug 15,2023
General Description
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is a synthetic compound derived from oats. It has several applications in skin therapy. It has potent anti-inflammatory properties, inhibiting mast cell degranulation and reducing the secretion of pro-inflammatory cytokines. These properties make it valuable for treating chronic pruritus and inflammatory skin conditions like eczema and psoriasis. Additionally, it exhibits cancer cell-inhibitory effects by suppressing MMP-9 expression and cell invasion in breast cancer cells. Furthermore, it shows promise in preventing skin aging by inhibiting ROS production and MMP expression, and promoting wound healing by facilitating cell proliferation and collagen synthesis. Overall, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid holds significant potential for various skin treatment applications.

Figure 1. 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
Activities
Anti-inflammatory
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is a synthetic analog of avenanthramides, which are active compounds found in oats known for their anti-inflammatory effects. In the context of chronic pruritus treatment, the potential of avenanthramides to disrupt mechanisms underlying itchiness was explored. A specific focus was placed on 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid and its interaction with the neurokinin-1 receptor (NK1R) and its ability to inhibit mast cell degranulation. Mast cells play a crucial role in immune responses and allergic reactions. When mast cells are activated, they release various mediators that can induce itchiness and inflammation, contributing to the development and persistence of chronic pruritus. In this study, it was discovered that it has the ability to interact with NK1R, a receptor involved in transmitting itch signals to the central nervous system. By binding to NK1R, it effectively inhibits mast cell degranulation, thereby reducing the release of itch-inducing mediators. Furthermore, it demonstrates additional anti-inflammatory properties. It influences inflammatory processes by decreasing the secretion of interleukin-6, a pro-inflammatory cytokine implicated in cutaneous inflammatory responses. By modulating inflammatory signaling, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid may contribute to a reduction in inflammation-related itch. 1
Cancer cell inhibitory
Previous studies have shown that 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid exhibits strong inhibition of nuclear factor-kappa B (NF-κB), which plays a crucial role in cancer cell invasion. In the present study, researchers investigated the effects of it on MMP-9 expression and cell invasion in MCF-7 human breast cancer cells. It was found that when the cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), an inducer of MMP-9 expression and cell invasion, both MMP-9 expression and cell invasion were increased. However, when it was added to the TPA-treated cells, these inductions were significantly suppressed. Further analysis revealed that it also inhibited the activation of mitogen-activated protein kinase (MAPK), a signaling pathway involved in cell proliferation and survival. This, in turn, led to the suppression of NF-κB and activator protein-1 (AP-1) activations, two key transcription factors associated with cancer progression, in the TPA-treated MCF-7 cells. Overall, the results suggest that 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid can effectively inhibit TPA-induced MMP-9 expression and cell invasion in breast cancer cells. This inhibitory effect is mediated through the suppression of the MAPK/NF-κB and MAPK/AP-1 pathways. 2
Anti-photoageing
The inhibitory effects of 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid on the UVB-induced production of reactive oxygen species (ROS) and the expression of matrix metalloproteinases (MMPs). Our study focused on human dermal fibroblasts subjected to UVB irradiation as the experimental model. Utilizing Western blot and real-time PCR analyses, we observed that it effectively suppressed the expression of UVB-induced MMP-1 and MMP-3. Additionally, it exhibited significant inhibition of ROS generation in fibroblasts following UVB exposure. Furthermore, it attenuated the UVB-induced phosphorylation of MAPKs and the activation of NF-κB and AP-1. Mechanistically, it exerted its regulatory effects on UVB-irradiated MMP expression by restraining the ROS-mediated activation of MAPK/NF-κB and AP-1 signaling pathways. Our comprehensive findings suggest that 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid holds promising potential as a candidate for the prevention of UV light-induced cutaneous photoageing. 3
Applications
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid is a compound that has shown great potential in the field of skin therapy. With its unique properties, it has been utilized for various applications in skin treatment. One of the main uses of it is in the prevention and treatment of skin aging. It has been found to inhibit the production of reactive oxygen species (ROS) and the expression of matrix metalloproteinases (MMPs), which are key factors in the aging process. By suppressing these factors, it helps to maintain the integrity and youthful appearance of the skin. Moreover, it has demonstrated anti-inflammatory properties, making it effective in treating skin conditions such as eczema and psoriasis. It helps to reduce redness, itching, and inflammation, providing relief to individuals suffering from these conditions. Additionally, it has been investigated for its wound healing properties. Studies have shown that it accelerates the healing process by promoting cell proliferation and collagen synthesis. This makes it a valuable component in topical formulations for wound care. Overall, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid holds promise as a versatile compound for skin therapy, offering benefits in anti-aging, anti-inflammatory, and wound healing applications. Further research and development in this field may uncover even more potential uses for this compound in the future. 4
Reference
1. Lotts T, Agelopoulos K, Phan NQ, Loser K, Schmaus G, Luger TA, St?nder S. Dihydroavenanthramide D inhibits mast cell degranulation and exhibits anti-inflammatory effects through the activation of neurokinin-1 receptor. Exp Dermatol, 2017, 26(8):739-742.
2. Lee YR, Noh EM, Oh HJ, Hur H, Kim JM, Han JH, Hwang JK, Park BH, Park JW, Youn HJ, Jung SH, Kim BS, Jung JY, Lee SH, Park CS, Kim JS. Dihydroavenanthramide D inhibits human breast cancer cell invasion through suppression of MMP-9 expression. Biochem Biophys Res Commun, 2011, 405(4):552-557.
3. Kim JM, Noh EM, Kwon KB, Hwang BM, Hwang JK, You YO, Kim MS, Lee W, Lee JH, Kim HJ, Kim JS, Lee YR. Dihydroavenanthramide D prevents UV-irradiated generation of reactive oxygen species and expression of matrix metalloproteinase-1 and -3 in human dermal fibroblasts. Exp Dermatol, 2013, 22(11):759-761.
4. Tourlaki A, Genovese G, Consonni D, Brambilla L. Efficacy of a detergent combined with a moisturizer for the treatment of pruritus associated with xerosis in an elderly population affected by Kaposi's sarcoma. G Ital Dermatol Venereol, 2020, 155(4):487-491.
- Related articles
- Related Qustion
- 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid: Preparation Method, Natural Sources and Potential in Skin Therapy Mar 13, 2024
Efficiently synthesized, 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid from oats offers diverse biological benefits and shows promise in skincare, inhibiting UV-induced skin damage and aging.
- 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid—a therapeutic treatment for allergic reaction Dec 23, 2019
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid, also called hydroxyphenyl propamidobenzoic acid, is a good anti-irritant ingredient effectively reduces skin itching.
2,2-Dimethyl-1,3-propanediol exhibits the barocaloric effect, undergoing solid-to-liquid phase transition with pressure changes. It is used in solid-state cooling, as a refrigerant.....
Aug 14,2023APIRuthenium is a rare transition metal with unique properties, used in nuclear reprocessing and cancer therapy.....
Aug 15,2023API2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
697235-49-7You may like
2-(3-(4-hydroxyphenyl)propanamido)benzoic acid manufacturers
- 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
-

- $0.00 / 1KG
- 2025-12-17
- CAS:697235-49-7
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 500kg/month
- 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
-
- $0.00 / 1kg
- 2025-12-17
- CAS:697235-49-7
- Min. Order: 1kg
- Purity: 99%min
- Supply Ability: 2000kgs
- 2-(3-(4-hydroxyphenyl)propanamido)benzoic acid
-

- $0.00 / 1kg
- 2025-12-15
- CAS:697235-49-7
- Min. Order: 0.001kg
- Purity: 99.99%
- Supply Ability: 2000000t