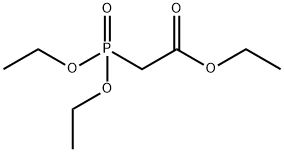
Synthesis and Wittig reaction of Triethyl phosphonoacetate
Dec 16,2025
Triethyl phosphonoacetate, a transparent and colorless liquid under ambient conditions, is insoluble in water but miscible with most organic solvents such as ethyl acetate and dichloromethane. As an important Wittig-Horner reagent, Triethyl phosphonoacetate is utilized in intramolecular Heck-type cyclization and isomerization reactions within Horner-Wadsworth-Emmons reactions, and also serves as a key intermediate for the synthesis of vitamin derivatives, pharmaceuticals, and natural compounds like insect pheromones.
Synthesis
A literature report describes an efficient synthetic method for Triethyl phosphonoacetate using diethyl phosphite and ethyl chloroacetate as starting materials, where the addition of a base (including but not limited to strong alkoxides such as sodium methoxide, ethoxide, propoxide, butoxide, or tert-pentoxide) facilitates the conversion of the P–H bond to a P–C bond via an SN2 mechanism, enabling nucleophilic attack on the C–Cl bond of ethyl chloroacetate. The use of polar aprotic solvents (including but not limited to toluene, acetonitrile, dichloromethane, tetrahydrofuran, acetone, diethyl ether, ethanol, or cyclohexane) enhances the SN2 reaction through solvation effects, effectively shortening the reaction time and improving the selectivity for Triethyl phosphonoacetate, yielding the product in 92% yield and 99.8% purity after only 0.5 hours at room temperature, with the advantages of low energy consumption, high efficiency, and straightforward operational steps. [1]
Wittig reaction

Figure1 : Wittig reaction of Triethyl phosphonoacetate
Under an argon atmosphere at 0°C, Triethyl phosphonoacetate (4.40 mL, 22.0 mmol) was added to a suspension of sodium hydride (55% dispersion in oil, 0.9165 g, 21.0 mmol) in tetrahydrofuran (25 mL), and the resulting mixture was stirred at this temperature for 20 minutes. A solution of 4-methoxybenzaldehyde (2.7232 g, 20.0 mmol) in THF (16.7 mL) was then added dropwise, followed by stirring as the reaction warmed from 0°C to room temperature over 30 minutes. After quenching with brine (20 mL), the mixture was concentrated under reduced pressure, extracted three times with dichloromethane, dried over anhydrous sodium sulfate, and concentrated again. The crude product was purified by silica gel column chromatography (hexane:ethyl acetate = 10:1) to afford (E)-ethyl 3-(4-methoxyphenyl)prop-2-enoate, demonstrating the effective role of Triethyl phosphonoacetate as a key reagent in this Horner–Wadsworth–Emmons olefination. [2]
Chemical Applications
Triethyl phosphonoacetate is employed in Horner-Wadsworth-Emmons reactions, intramolecular Heck-type cyclizations, isomerization processes, Tsuji-Trost reactions, intramolecular aryne-alkene transformations, and aldehyde synthesis. As a significant Wittig-Horner reagent, Triethyl phosphonoacetate also functions as a plasticizer, flame retardant, and extractant, in addition to serving as a key intermediate in the production of vitamin-based compounds, pharmaceuticals, and natural substances such as insect pheromones.
Synthesis of Ambrisentan
Ambrisentan is an endothelin-1 receptor antagonist, for which 2-hydroxy-3-methoxy-3,3-diphenylpropanoic acid serves as a key synthetic intermediate. A novel synthetic approach to this intermediate has been reported, wherein benzophenone undergoes a Wittig-Horner reaction with Triethyl phosphonoacetate to afford ethyl 3,3-diphenylacrylate. Using methanol as solvent, this acrylate ester then undergoes electrophilic addition with N-bromosuccinimide (NBS) to yield ethyl 2-bromo-3-methoxy-3,3-diphenylpropanoate. Subsequent esterification with acetic acid gives ethyl 2-acetoxy-3-methoxy-3,3-diphenylpropanoate, which, without isolation, is directly hydrolyzed to obtain the target 2-hydroxy-3-methoxy-3,3-diphenylpropanoic acid with an overall yield of 75%. This streamlined process, utilizing the readily available Triethyl phosphonoacetate, avoids the Darzens condensation reaction, and is characterized by inexpensive starting materials, simple operations, and suitability for industrial-scale production. [3]
Synthesis of β-ketophosphonates
A practical synthesis of β-ketophosphonates from Triethyl phosphonoacetate has been reported in the literature, wherein acylation of Triethyl phosphonoacetate with carboxylic acid chlorides is carried out in the presence of a magnesium chloride–triethylamine system, followed by decarbethoxylation. Specifically, a mixture of the phosphonate, triethylamine, and MgCl₂ in dry toluene is prepared at room temperature, after which the acid chloride is added at 0 °C; the resulting suspension is stirred for 6 h at room temperature to form the acylated adduct 2, which is then hydrolyzed catalytically with p-TsOH in water to afford the β-ketophosphonates in good yield. The use of LiCl in place of MgCl₂ led to diminished yield (61% for product). [4]
Reference
[1] Gao X, Huang S C, Zhao M M, et al. A method for high-efficiency synthesis of triethyl phosphonoacetate: CN202410731417.9[P].
[2] Goto, Toshihito; et al, I2-mediated convenient ring-opening of simple gem-difluorocyclopropanes, Organic & Biomolecular Chemistry 2025, 23, 3163-3170.
[3] Jia S, Zhang C C, Wang S, et al. Novel synthesis of a key intermediate of ambrisentan[J]. Chinese Journal of Pharmaceuticals, 2021, 52: 207-210.
[4] Peter Fantke, Dae Young Kim, Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry, Sustainable Chemistry and Pharmacy, 2015, 1:1-8.
- Related articles
- Related Qustion
Sulfanilamide, one of the earliest synthetic antimicrobial agents, holds a foundational place in medicinal chemistry because it introduced the concept of selectively inhibiting bacterial metabolism.....
Dec 15,2025API2-(Perfluorohexyl)ethyl methacrylate finds applications in textiles, coatings, and the preparation of fluorinated surfactants.....
Dec 16,2025APITriethyl phosphonoacetate
867-13-0You may like
Triethyl phosphonoacetate manufacturers
- Triethyl phosphonoacetate
-

- $0.00 / 1KG
- 2025-12-16
- CAS:867-13-0
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Triethyl phosphonoacetate
-

- $10.00 / 1KG
- 2025-12-11
- CAS:867-13-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Triethylphosphonoacetat
-
- $8.00 / 1KG
- 2025-09-25
- CAS:867-13-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available