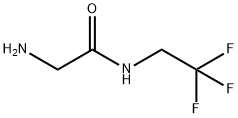
2-amino-N-(2,2,2-trifluoroethyl) acetamide: Preparation and Appication
Jan 5,2023
General description
2-amino-N-(2,2,2-trifluoroethyl) acetamide, which is usually stable in the form of salt, is a key intermediate in the synthesis of fluralaner, a new broad-spectrum veterinary insecticide. Flurellana is an isozoline insecticide, which acts by interfering with gamaminobutyric acid (GABA) gated chloride ion channels. Compared with phenylpyrazoles, cyclopentadienes and macrolides, flurellana has significant differences in molecular structure, action site, selectivity and cross-resistance. It is safe for mammals and has high insecticidal activity.
Preparation
The method of preparing 2-amino-N-(2,2,2-trifluoroethyl) acetamide and its salt is to react the glycine protected by N-phthalyl group with trifluoroethylamine or its salt to form an amide, and remove the protective group under the action of hydrated fibril to obtain the crude product of 2- amino-N-(2,2,2-trifluoroethyl) acetamide. The crude product can be salted with acid to get a salt of 2-amino-N-(2,2, 2-trifluoroethyl) acetamide, and finally add a base to dissociate pure 2-amino-N-(2,2, 2-trifluoroethyl) ethylkuroamine. The deprotective reaction reagent used in the invention has the advantages of cheap price, simple operation, no need to use flammable and explosive hydrogen, and the reaction temperature can be realized from room temperature to solvent reflux temperature. The reaction condition is mild, and is very suitable for the production of industrial devices [1].
The 2-amino-N-(2,2,2-trifluoroethyl)acetamide or salts thereof is a key intermediate in the field of pharmaceuticals and agrochemicals for preparing many active ingredients. The Japanese patent no. JP5652628B2(henceforth ‘628) disclosed a method for producing 2-amino-N-(2,2,2-trifluoroethyl) acetamide or a salt thereof in two steps i) by reacting chloroacetyl chloride with 2,2,2-trifluoroethylamine in presence of inorganic base such as sodium hydroxide, water ii) treating with aqueousammonia in presence of methyl tert-butyl ether in an autoclave under pressure. The above reaction involves extensive reaction conditions and further leads to formation of dimer impurity N-(2,2,2-trifluoroethyl)-2-{[(2,2,2-trifluoro-ethylcarbamoyl)-methyl]-amino} acetamide. The dimer is resulting of sequential reaction of unreacted 2-chloro-N-(2,2,2-trifluoroethyl) acetamide. It involves further purification there by reduces the yield and increases overall cost [2].
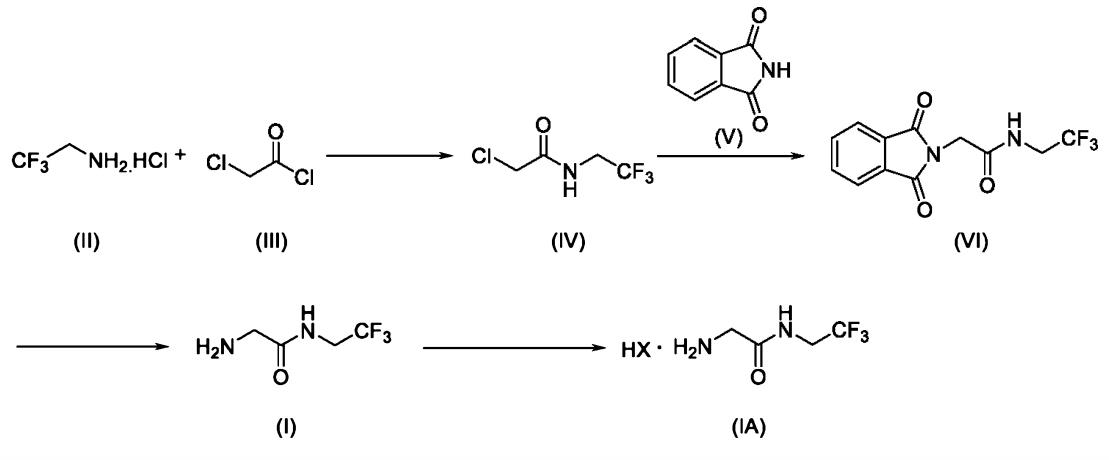
Fig. 1 The synthetic method of 2-amino-N-(2,2,2-trifluoroethyl) acetamide.
Application
2-amino-N-(2,2,2-trifluoroethyl) acetamide, which is usually stable in the form of salt, is a key intermediate in the synthesis of fluralaner, a new broad-spectrum veterinary insecticide. Fluralaner belongs to the class of isowarlinoid insecticides, which exerts its effect by interfering with gamaminobutyric acid (GABA) gated chloride ion channels. Compared with phenylpyvawans, cyclopentadienes and macrolides, flurrana has significant differences in molecular structure, action site, selectivity and cross-resistance. It is safe for mammals and has high insecticidal activity.
Backwoods insecticides are a new class of highly effective insecticides that cause nervous system overexcitation and death in parasites by interfering with gamma-aminobutyric acid (GABA) gated chloride ion channels. Compared with traditional insecticides, heterophobic and heterophobic insecticides have obvious differences in target, molecular structure and selectivity. Forest insecticides are used in pet cats and dogs for the treatment of parasites such as fleas and splenworms. This patent relates to the synthesis of 2-amino-N-(2,2,2-trifluoroethyl) acetamide as an intermediate for fluoroethyl insecticide [3].
Fluralaner was marketed as a veterinary drug (BravectoTM) in Germany, Spain, Italy, France, the Netherlands and the United Kingdom in 2014. Fluralaner is mainly used to kill animals in vitro and in vivo. In July 2016, the FDA approved Merck's application for fleas and ticks in cats and dogs for up to 12 weeks at a time. Studies have shown that fluralaner is a class of highly effective insecticidal active compounds, which can cause nervous system overexcitation and death by interfering with γ-amino butyric acid (GABA) gate channels. As a new efficient GABA-controlled chloride channel jammer, fluralaner is significantly different from phenylpyrazoles, cyclopentadienes and macrolides in terms of molecular structure, site of action, selectivity and cross-resistance. Its central pentadiene insecticides Dieldrin and Lindane are the first discovered insecticides that act on GABa-gated chloride ion channels. However, these two insecticides are banned in agricultural production worldwide due to their high residues, easy environmental pollution and harm to human health, as well as the increase of resistance of agricultural pests. The invention relates to the synthesis of fluralaner, The key step of synthesis involves in Zn(OTf)2/hydroxyamine hydrochloride 4-(5-(3,5-dichlorobenzyl)-5-(trifluoromethyl)-4,5-dihydroisooxazole-3- base)-2-methyl benzonitrile and 2-amino-N (2,2,2-trifluoroethyl) acetamide reaction to achieve the preparation of fluralaner [4].
References
[1] Li Q, Gu D, Li L, Fan W. Preparing 2-amino-N-(2,2,2-trifluoroethyl)acetamide comprises carrying out amidation reaction of phthalylglycine and trifluoroethylamine and carrying out deprotection reaction, and purifying and adding alkali[P]. Famingzhuanli Shengqing, 2017, 107353222.
[2] Nambiar S, Gilla G, Nageshwar G, Mokal RA. Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide (salt) for preparing fluralaner, involves preparing 2-amino-N-(trifluoroethyl)acetamide using 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-N-(2,2,2-trifluoroethyl) and adding acid [P]. PCT Int. Appl., 2020, 2020222158.
[3] Li B, Jiang Q, Zhu J. Preparation of isoxazoline insecticide comprises reacting carboxylic acid derivative and hydroxylamine hydrochloride, reacting with 2-amino-N-(2,2,2-trifluoroethyl)acetamide and N-halosuccinimide, and elimination and cyclization reacting[P]. Famingzhuanli Shengqing, 2019, 109879826.
[4] Yang X, Zhu C. Preparing fluralaner comprises e.g. reacting 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethan-1-one with 4-acetyl-2-methylbenzonitrile, treating with dehydrating reagent, reacting with hydroxylamine hydrochloride, and reacting with 2-amino-N-(2,2,2-trifluoroethyl)acetamide[P]. Famingzhuanli Shengqing, 2021, 113512007.
- Related articles
- Related Qustion
- 2-Amino-N-(2,2,2-trifluoroethyl)acetamide: Properties, Applications in Preparation of Fluralaner and Its Green Preparation Apr 16, 2024
2-Amino-N-(2,2,2-trifluoroethyl)acetamide is a crucial intermediate in drug development, showcasing versatility and sustainability in pharmaceutical industries.
GSK-J4 is a potent dual inhibitor of H3K27me3/me2-demethylases?JMJD3/KDM6B?and?UTX/KDM6A?with?IC50s of 8.6 and 6.6 μM, respectively.....
Jan 5,2023APIFluorescein sodium is mainly used as a tool of diagnosis in the fields of optometry and ophthalmology. Fluorescein angiography stains the blood vessels of the retina and iris to give out a detailed image of the posterior view of the eye.....
Jan 5,2023Biochemical Engineering2-amino-N-(2,2,2-trifluoroethyl)acetamide
359821-38-8You may like
2-amino-N-(2,2,2-trifluoroethyl)acetamide manufacturers
- 2-Amino-N-(2,2,2-trifluoroethyl)acetamide
-

- $3.00 / 25KG
- 2025-10-13
- CAS:359821-38-8
- Min. Order: 0.1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 2-Amino-N-(2,2,2-trifluoroethyl)acetamide
-

- $0.00 / 25KG
- 2025-07-25
- CAS:359821-38-8
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 10000KGS