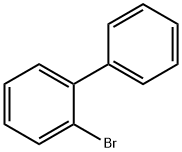
2-Bromobiphenyl: Organic Synthesis Intermediate & Substrate
Dec 23,2025
2-bromobiphenyl is used as an intermediate of organic synthesis and pharmaceutical. At room temperature, it typically exists as a solid but may also be found as a liquid if the temperature is slightly elevated. 2-Bromobiphenyl appears as a colorless to pale yellow solid or liquid, depending on the specific arrangement and conditions. It has a shiny, crystalline texture when in solid form.

2-bromobiphenyl applied in chemical synthesis
2-bromobiphenyl is synthesized from a crude 2-aminobiphenyl: 16.5 g (97.51 mmol) of 2-aminobiphenyl and 27.9 g (195.01 mmol) of copper bromide were added to 200 ml of acetonitrile and stirred at 0°C for 30 minutes. 17.4 mL (146.26 mmol) of tert-butylnitrilate was slowly added dropwise. Raise the temperature to 60℃ and react for 6 hours. After confirming the completion of the reaction. Cool to room temperature and quench by adding 100 mL of water. Extract it three times with 100 mL of water and 100 mL of ethyl acetate. The collected organic layer is dried with magnesium sulfate and the solvent is evaporated. The obtained residue was subjected to silica gel chromatography using a 1:9 mixed solution of methylene chloride and n-hexane as an eluent to obtain 11.0 g (48.4%) of the target compound 2-bromobiphenyl.[1]
10.8 g (51.91 mmol) of 9,9-dimethyl-9H-fluorene-2-amine and 13.6 g (141.57 mmol) of sodium tert-butoxide were added to 120 mL of toluene and stirred for 30 minutes to dissolve. Add 11.0 g (17.19 mmol) of 2-bromobiphenyl dropwise here and stir at 50°C for 1 hour. A solution of 0.27 g (0.47 mmol) of bis(dibenzylideneacetone)palladium (0) dissolved in 0.57 mL (1.18 mmol) of tri-tert-butylphosphine was added to the reaction solution, and then reacted at 100°C for 18 hours. After confirming the consumption of 2-bromobiphenyl, cool to room temperature, filter through silica, and distill the filtrate under reduced pressure. The obtained residue was subjected to silica gel chromatography using a 1:4 mixed solution of methylene chloride and n-hexane as an eluent to obtain the target compound N-([1,1'-biphenyl]-2-yl)9,9-dimethyl-9H-fluorene. -2-amine 9.4g (55.1%) was obtained.
A General and Special Catalyst for Suzuki–Miyaura Coupling Processes
Since its discovery, the Suzuki–Miyaura cross-coupling reaction has evolved into one of most powerful carboncarbon bond-forming transformations and has shown increasing utility in synthetic organic chemistry. Recent advances in novel catalyst development have enabled this transformation to be applied with a broad substrate scope, a wide functional group tolerance, and low catalyst loadings. Palladium catalysts derived from electron-rich and sterically demanding ligands, such as trialkyl monophosphanes, dialkyl aryl monophosphanes, dialkyl biaryl monophosphanes, N-heterocyclic carbenes, and other ligands, have been particularly effective. Ligands 1 a–d were applied in the palladium-catalyzed Suzuki–Miyaura coupling of sterically congested arylboronic acids. The cross-coupling between 2,4,6-triisopropylphenylboronic acid and 2-bromobiphenyl was chosen as the model system. Besides ligands 1 a–d, various structurally diverse ligands were also examined for comparison. The reactions were carried out at 100 °C in toluene for 12 hours with [Pd2(dba)3] (0.5 mol %) as the catalyst precursor. Whereas low conversions (<20 %) were generally observed in this challenging cross-coupling reaction with ligands such as SPhos, RuPhos, XPhos, Ad2PnBu PPh3 , Cy3P, tBu3P, and ligands 1 a–c, it is gratifying to report that BI-DIME provided a rapid conversion and an excellent yield (89 %). Ligand screening for Suzuki–Miyaura coupling of 2,4,6-triisopropylphenylboronic acid and 2-bromobiphenyl.[2]
The reactions were heated at 100 °C in toluene (2 mL) for 12 h with 2-bromobiphenyl (1 mmol), 2,4,6-triisopropylphenyl boronic acid (1.5 mmol), and K3PO4 (3 mmol) in the presence of [Pd2dba3] (0.5 mol %) and ligand (2 mol %). HPLC assay yields were averaged from two runs. The high effectiveness of ligand 1 d observed in the coupling of 2,4,6-triisopropylphenylboronic acid with 2-bromobiphenyl encouraged us to study the substrate scope in the Suzuki–Miyaura coupling of sterically congested di-ortho-substituted arylboronic acids. As illustrated, ligand 1 d is capable of catalyzing the coupling of hindered arylboronic acids or esters to form tri-ortho-substituted or tetra-ortho-substituted biaryl products in excellent yields. A range of ortho-substituted aryl bromides were coupled with 2,4,6-triisopropylphenylboronic acid to afford the corresponding tri-ortho-substituted biaryl products in very high yields. Biaryl monophosphorus ligands containing a 2,3-dihydrobenzo[d][1,3]oxaphosphole framework are highly effective for the palladium-catalyzed Suzuki–Miyaura cross-coupling reactions of a wide range of substrates.
References
[1]BPC - KR2023/133584, 2023, A Location in patent: Paragraph 0125-0128
[2]Tang, W., Capacci, A. G., Wei, X., Li, W., White, A., Patel, N. D., Savoie, J., Gao, J., Rodriguez, S., Du, B., Haddad, N., Lu, B. Z., Krishnamurthy, D., Yee, N. K., Senanayake, C. H. (2010). A general and special catalyst for Suzuki-Miyaura coupling processes. Angewandte Chemie International Edition, 49(34), 5879-5883.
- Related articles
- Related Qustion
- Synthesis and Application of 2-Bromobiphenyl Aug 5, 2022
2-Bromobiphenyl is an important pharmaceutical intermediate and organic intermediate.
2-Amino-5-nitrothiazole (heterocyclic amine) is a dye precursor, and carcinogenic in male rats; it serves as a head group for active nitazoxanide analogues.....
Dec 23,2025Chemical Reagents2-Bromobiphenyl
2052-07-5You may like
- 2-Bromobiphenyl
-
- $39.00 / 100mg
- 2025-12-22
- CAS:2052-07-5
- Min. Order:
- Purity: 99.74%
- Supply Ability: 10g
- 2-Bromobiphenyl
-
- $0.00 / 1GK
- 2025-12-22
- CAS:2052-07-5
- Min. Order: 10g
- Purity: 99.50%
- Supply Ability: 10TONS
- 2-Bromobiphenyl
-
- $200.00 / 1KG
- 2025-09-25
- CAS:2052-07-5
- Min. Order: 1KG
- Purity: 99%, 99.5% Sublimated
- Supply Ability: g-kg-tons, free sample is available