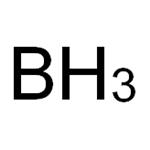
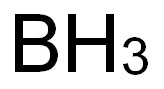A non metallic element-Boron
Feb 27,2024
Metals, nonmetals, or metalloids
The elements can be classified as metals, nonmetals, or metalloids. Metals are good conductors of heat and electricity and are malleable (they can be hammered into sheets) and ductile (they can be drawn into wire). Most metals are solids at room temperature, with a characteristic silvery shine (except for mercury, a liquid). Nonmetals are (usually) poor conductors of heat and electricity and are not malleable or ductile; many elemental nonmetals are gases at room temperature, while others are liquids and solids. The metalloids are intermediate in their properties. They are more like nonmetals in their physical properties, but under certain circumstances, several of them can be made to conduct electricity. These semiconductors are essential in computers and other electronic devices.
On many periodic tables, a jagged black line (see figure below) along the right side of the table separates the metals from the nonmetals. The metals are to the left of the line (except for hydrogen, which is a nonmetal), the nonmetals are to the right, and the elements immediately adjacent to the line are the metalloids.

Boron
Group 3A (or IIIA) of the periodic table includes the metalloid boron (B), as well as the metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Boron forms mostly covalent bonds, while the other elements in Group 3A form mostly ionic bonds.
Boron is a nonmetallic element and the only nonmetal element in group 13 of the periodic table elements. Boron is electron-deficient, possessing a vacant p-orbital. It has several forms, the most common of which is amorphous boron, a dark powder, unreactive to oxygen, water, acids, and alkalis. It reacts with metals to form borides. Boron is classified as a metalloid, having properties of both metals and nonmetals: it conducts electricity at high temperatures, but at room temperature, it is an insulator. Many boron salts emit a green color when heated.
Pure crystalline boron is a black, lustrous semiconductor; i.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. It is hard enough (9.3 on the Mohs scale) to scratch some abrasives, such as carborundum, but too brittle for use in tools. It constitutes about 0.001 percent of the weight of Earth’s crust.
Scientists have known for many years that boron is essential for healthy bones. Boron plays a vital role in osteogenesis, and its deficiency has been shown to adversely impact bone development and regeneration. Boron influences the production and activity of steroid hormones, actions via which this trace mineral is involved in preventing calcium loss and bone demineralization. Boron supplementation has repeatedly been shown to markedly reduce urinary excretion of calcium and magnesium and increase serum levels of estradiol and calcium absorption in peri- and postmenopausal women[1]. Boron also beneficially impacts vitamin D utilization. Supplementation with boron stimulates bone growth in vitamin-D-deficient animals and alleviates dysfunctions in mineral metabolism characteristic of vitamin-D deficiency.
References
[1] T. Swager, & S. Luppino. “Nothing Boring about this Borylation.” Synfacts 61 1 (2015): 0266–0266.
- Related articles
- Related Qustion
- Boron: Chemical information, Major minerals, Chemical reactions and Major uses May 27, 2024
Boron exist as two natural stable isotopes, 11B (80.1%) and 10B (19.9%).
- Boron: An Element with Exceptional Chemistry Apr 7, 2024
Unlike its group 13 neighbors, boron exhibits behaviors and forms compounds that are not quite metallic and yet not fully nonmetallic.
- How many valence electrons does boron have? Mar 12, 2024
Boron has 3 valence electrons. The number of electrons in the outermost (or) valence shell layer of an atom is called valence electrons.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringH2CO3 is a carbon-containing compound often found in solutions of carbon dioxide in water. This article will collate the chemical name, uses and properties of H2CO3.....
Feb 27,2024Inorganic chemistry