A novel synthesis of 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane
Apr 18,2025
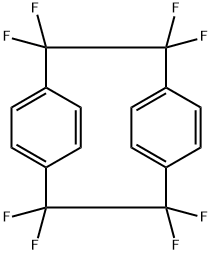
1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane, also known as Parylene AF4, is an important compound used to test theories of bonding, ring strain, and delta-electron interactions. It is also a precursor for the chemical vapor deposition (CVD) of thin film polymers (poly(p-xylene)). These poly(p-xylene) are well suited for use as conformal coatings in a wide range of applications in the electronics, semiconductor, automotive, and medical industries.
An important aspect of the synthesis of 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane is that the reaction must be performed under highly dilute conditions. The most common approach is to generate a p-xylene intermediate, which then dimerizes to form the para-cycloarene, as shown in the general synthesis of AF4 in Scheme 1.
![1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane synthesis 1 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane synthesis 1](/NewsImg/2025-04-18/6388058708467047983386103.png)
In such processes, high dilution conditions are often required to provide an optimal kinetic environment in which the unimolecular cyclization of the intermediate radical 3 can effectively compete with the unwanted bimolecular oligomerization reaction. Although gram quantities of AF4 were first prepared using the traditional high dilution method, this also hindered the large-scale scale-up of the process.
Now, researchers have found that 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane can be synthesized without using high dilution techniques. The study found that heating a mixture of 4 equivalents of zinc powder and 0.35M p-bis(chlorodifluoromethyl)benzene in dimethylacetamide to 100°C can produce AF4 in 60% yield, accompanied by some obvious oligomeric byproducts, but almost no insoluble polymers are formed.
![1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane synthesis 2 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane synthesis 2](/NewsImg/2025-04-18/6388058709416402663750466.png)
References:
[1] WILLIAM R. DOLBIER. A Novel, Non-High-Dilution Method for Preparation of 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane[J]. Organic Letters, 2000, 2 13: 1867-1869. DOI:10.1021/ol005943f.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringParylene (poly-p-xylene) is a polymer of p-xylene, which is formed by thermal decomposition and polymerization of poly-p-xylene dimer (called di-p-xylene).....
Apr 18,2025APIYou may like
1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane manufacturers
- AF4
-
- $1.00 / 500g
- 2025-12-12
- CAS:3345-29-7
- Min. Order: 300g
- Purity: 99.8%
- Supply Ability: 20 TONS
- 2,2,3,3,8,8,9,9-Octafluorotricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene
-
![3345-29-7 2,2,3,3,8,8,9,9-Octafluorotricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene](/ProductImageEN1/2025-04/Small/3e49e06c-24ff-461e-9022-d9b3389258a6.gif)
- 2025-12-12
- CAS:3345-29-7
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Parylene AF4
-

- $0.00 / 100g
- 2025-12-11
- CAS:3345-29-7
- Min. Order: 10g
- Purity: 99%
- Supply Ability: ton