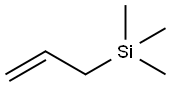
Allyltrimethylsilane: Reagent Applications and Polymerization/Catalytic Reaction Studies
May 6,2025
Allyltrimethylsilane is a general reagent to introduce allyl groups across acid chlorides, aldehydes, ketones, iminium ions, enones, and for cross-coupling with other carbon electrophiles. It is used as a reagent in Hosomi−Sakurai reaction.

Homo- and copolymerizations with silicon-containing monomers and metallocene-based polymerization catalysts
In addition to the Ziegler–Natta systems, there are also some reports of homo- and copolymerizations with silicon-containing monomers with metallocene-based polymerization catalysts. In 1994, Zeigler et al. employed different metallocenes for the preparation of isotactic, syndiotactic, and atactic poly(allyltrimethylsilane). Polymerization of allyltrialkylsilanes with a higher steric demand leads to a decrease in polymer yield and molar mass. With allyltriphenylsilane on a MAO-activated metallocene, only oligomers can be obtained, whereas with allyltrimethylsilane, and the same catalyst under the same polymerization conditions, a true polymer is formed. Usage of metallocenes like rac-Et(Ind)2ZrCl2/MAO leads to an activity decrease with increasing amounts of allyl- and vinyltrimethylsilane. The molecular weight of the poly(allyltrimethylsilane-co-ethene) is hardly affected by increasing amounts of silane comonomer. The lower molar mass is assumed to be a result of a charge build-up from silicon in the transition state, leading to a faster β-hydride elimination. NMR spectroscopic end-group analysis confirmed this possible influence of silicon because of a high incorporation degree of the silane monomers as terminal vinylidene groups with a Cp2ZrCl2/MAO catalytic system. In the case of a rac-Et(Ind)2ZrCl2/MAO catalyst, however, the end-group analysis reveals an increased incorporation degree of allyltrimethylsilane with an increasing amount of silane comonomer in the feed. In this case, allyltrimethylsilane acts as a real comonomer. The sharp decrease in molar mass must therefore be due to a different effect of the silane comonomer.[1]
Another promising approach to copolymerize allyltrimethylsilane and ethene was performed by Liu and Nomura. Therefore, nonbridged half-titanocenes were used and copolymers without the drastic decrease in molar mass with increasing amount of silane comonomer as was seen for zirconocene complexes such as Cp2ZrCl2 or rac-Et(Ind)2ZrCl2 were obtained. The incorporation level of the silane monomer reach values up to 60 mol% in the case of catalyst and a concentration of the silane monomer in the feed of 1.05 mol l−1. The same catalysts can also effectively incorporate vinyltrimethylsilane into a PE backbone. Until this publication, there were no reports of ordinary metallocenes being able to coordinate and as a result polymerize or copolymerize this bulky silane monomer. Catalyst shows the best activities and the highest incorporation levels of the silane comonomer. At a silane concentration of 1.1 mol l−1 and an ethene pressure of 6.1 bar an activity of 3730 kgpolymer molTi−1 h−1 is obtained. The corresponding copolymer has an Mn of 573 000 g mol−1 and a silane content of 11.9 mol%. Upon increasing the concentration of silane comonomer to 2.3 mol l−1, the activity decreases to 820 kgpolymer molTi−1 h−1, the molar mass to an Mn of 304 000 g mol−1, and the silane content in the copolymer increases to 21.4 mol%. This demonstrates a decrease in molar mass with an increasing amount of vinyltrimethylsilane in the feed, but it is not as dramatic as in the case of the common metallocene polymerization catalysts and allyltrimethylsilane.
Highly Enantioselective Addition of Allyltrimethylsilane to Aldehydes
A general and highly enantioselective catalytic addition of inexpensive, nontoxic, air- and moisture-stable allyltrimethylsilane to aldehydes, the Hosomi–Sakurai reaction, is disclosed. Reported is the design and synthesis of a highly acidic imidodiphosphorimidate motif (IDPi), which enables this transformation, and converts various aldehydes at catalyst loadings ranging from 0.05 to 2.0 mol % with excellent yields and enantioselectivities. The enantioselective allylation of aldehydes to form homoallylic alcohols is one of the most frequently used carbon–carbon bond-forming reaction in chemical synthesis and, for several decades, has been a testing ground for new asymmetric methodology. However, a general and highly enantioselective catalytic addition of the inexpensive, nontoxic, air- and moisture-stable allyltrimethylsilane to aldehydes, the Hosomi–Sakurai1 reaction, has remained elusive. Reported herein is the design and synthesis of a highly acidic imidodiphosphorimidate motif (IDPi), which enables this transformation, thus converting various aldehydes with aromatic and aliphatic groups at catalyst loadings ranging from 0.05 to 2.0 mol % with excellent enantioselectivities. Our rationally constructed catalysts feature a highly tunable active site, and selectively process small substrates, thus promising utility in various other challenging chemical reactions.[2]
Despite significant effort, however, a general and broadly applicable allylation of aldehydes with allyltrimethylsilane as a highly attractive allyl-transfer reagent has proven to be extremely challenging. Previously developed variants exploiting cyclic alkoxy boranes (CAB) or titanium-based catalysts generate the corresponding homoallylic alcohols with moderate to good yields and enantioselectivities. These methods require exhaustive exclusion of air and moisture and are limited with regard to substrate diversity. Most importantly, and enhanced by the low nucleophilicity of allyltrimethylsilane, catalytic enantioselective Hosomi–Sakurai reactions struggle with highly efficient and competitive non-enantioselective background silylium catalysis. Attractive features of silylium-based Lewis acid organocatalysis include the in situ regeneration of the catalyst (“self-healing”) and relatively low catalyst loadings, thus suggesting a practical synthetic method for the allylation of aldehydes with allyltrimethylsilane. Our effort in catalyst design was quickly rewarded with a highly enantioselective organocatalytic addition of allyltrimethylsilane to aromatic aldehydes. IDPi catalysts proved to be general, and diverse homoallylic alcohols were obtained in good yields and high enantioselectivities. Aromatic aldehydes were converted at −78 °C with 0.5 mol % of IDPi bearing 2-naphthyl substituents in the 3,3′-positions of the BINOL backbones. In both cases, the configuration of the pre-existing stereogenic center was preserved. Our results show that confined organocatalysts with extreme acidity and steric demand can overcome current synthetic limitations and solve a long standing problem in chemical synthesis. Our designed high-performance organocatalysts enable the first general and enantioselective catalytic addition of allyltrimethylsilane to aldehydes.
References
[1]Schöbel, A., Winkenstette, M., Anselment, T. M. J., & Rieger, B. (2012). 3.24 - Copolymerization of Alkenes and Polar Monomers by Early and Late Transition Metal Catalysts. In Polymer Science: A Comprehensive Reference (Volume 3, pp. 779-823).
[2]Kaib, Philip S J et al. “Extremely Active Organocatalysts Enable a Highly Enantioselective Addition of Allyltrimethylsilane to Aldehydes.” Angewandte Chemie (International ed. in English) vol. 55,42 (2016): 13200-13203.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCiclopirox ethanolamine is a hydroxypyridone derivative, that is structurally unrelated to the common imidazole derivatives. Here summarized its Pharmacology.....
May 6,2025DrugsAllyltrimethylsilane
762-72-1You may like
Allyltrimethylsilane manufacturers
- Allyltrimethylsilane
-
- $85.00 / 5g
- 2025-12-12
- CAS:762-72-1
- Min. Order: 1g
- Purity: 0.98
- Supply Ability: 25kg
- Allyltrimethylsilane
-
- $0.00 / 25KG
- 2025-12-01
- CAS:762-72-1
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KGS
- Allyltrimethylsilane
-
- $8.00 / 1kg
- 2025-09-25
- CAS:762-72-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available