Pharmacological activity of ciclopirox ethanolamine
May 6,2025
Introduction
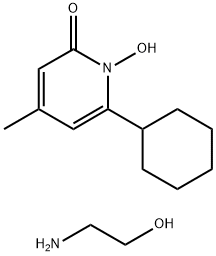
Ciclopirox ethanolamine (Figure 1) is a hydroxypyridone derivative, being a broad-spectrum antifungal, antibacterial, and anti-inflammatory agent. It acts by chelating polyvalent cations (such as aluminium and iron) that inhibits metal-dependent enzymes responsible for peroxides' degradation in fungal cells. The drug also modulates the activity of cytochromes and catalase, affects mitochondrial transport processes, interrupts energy production, and affects cell membrane permeability that impairs the transmembrane transport of nutrients. It is also speculated to be involved in the impairment of DNA repair mechanisms and mitosis.[1]

1. Antimicrobial Activity[2]
Ciclopirox ethanolamine, a substituted pyridone not related to the imidazole derivatives, is an antimycotic agent with activity against a broad spectrum of dermatophytes, yeasts, actinomycetes,molds and other fungi. Antibacterial activity has also been demonstrated, e.g. against Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa,and relevant Staphylococcus and Streptococcus species. Activity against mycoplasmas, trichomonads and chlamydias has also been reported. Ciclopirox ethanolamine is thought to exert its antifungal action by intracellular depletion of some essential substrates and/or ions caused by inhibition of their uptake.
Mechanism of Action
The primary site of action of ciclopirox ethanolamine appears to be the cell membrane. The drug accumulates in yeast cells to concentrations approximately 200 times higher than in the surrounding culture medium. In vitro tests with Candida albicans and Saccharomyces cerevisiae have shown that ciclopirox ethanolamine blocks transmembrane transport of radiolabelled leucine. At a concentration of 20 mg/L, which is the MIC of C. albicans. there is a greater than 90% inhibition of leucine accumulation into the intracellular amino acid pool. At higher concentrations, ciclopirox ethanolamine begins to alter the integrity of the cell membrane of sensitive organisms and results in leakage of potassium ions and other intracellular materials. Actions similar to the last mentioned have been reported with nystatin, clotrimazole and miconazole. Furthermore, biochemical tests suggested that ciclopirox may inhibit the arachidonic acid cascade and might thus be expected to have anti-inflammatory and antiallergic activity.
2. Anti-inflammatory Action of Ciclopirox ethanolamine
Fungal diseases of the skin may be complicated by the presence of inflammation. The anti-inflammatory activities of ciclopirox ethanolamine and other antifungal agents have been studied in a number of models. Inhibition of prostaglandin and leukotriene synthesis by ciclopirox ethanolamine in human polymorphonuclear cells was first described in 1983. Ciclopirox ethanolamine and ciclopirox acid significantly reduced arachidonic acid-induced ear edema (P < 0.05), as measured by percentage change from control inflamed ears. None of the other antifungal agents had a significant anti-inflammatory effect. The anti-inflammatory activities of ciclopirox ethanolamine and ciclopirox acid were similar to those of the reference anti-inflammatory agents indomethacin and desoximetasone.[3]
3. Anti-tumor activity
Hepatocellular carcinoma is one of the most common fatal malignancies worldwide. Thus far, the hepatocellular carcinoma prognosis has been bleak due to deficiencies in the identification and diagnosis of early hepatocellular carcinoma. Ciclopirox ethanolamine is a synthetic antifungal agentand has been considered as an anti-cancer candidate drug recently, though the detailed mechanisms related to its anti-cancer effect in hepatocellular carcinoma have not yet been revealed. Here, the researchers found that ciclopirox ethanolamine could inhibit proliferation in HCC cells but not in intrahepatic cholangiocarcinoma cells by arresting the cell cycle. Moreover, the anti-cancer effects of Ciclopirox ethanolamine in HCC cells were also attributed to ciclopirox ethanolamine-triggered ROS accumulation and DJ-1 downregulation. Additionally, ciclopirox ethanolamine could promote complete autophagic flux, which alleviated the anti-cancer effect of Ciclopirox ethanolamine in HCC cells,whereas the ROS scavenger (NAC) would attenuate ciclopirox ethanolamine-induced protective autophagy. Interestingly, ciclopirox ethanolamine could also induce glycogen clustering in HCC cells. Altogether, this study provides a new insight into the detailed molecular mechanisms of ciclopirox ethanolamine as an anti-cancer therapy and a strategy for treating hepatocellular carcinoma.[4]
Other study observed that ciclopirox ethanolamine displayed strong antitumorigenic properties in lung adenocarcinoma (LUAD) cells, inhibited LUAD proliferation, induced ROS production, caused DNA damage, and activated the ATR-CHK1-P53 pathway. Topoisomerase II alpha (TOP2A) is overexpressed in LUAD and associated with a poor prognosis. By analyzing differentially expressed genes (DEGs), TOP2A was significantly down-regulated in ciclopirox ethanolamine-treated LUAD cells. Furthermore, ciclopirox ethanolamine treatment substantially inhibited in vivo LUAD xenograft growth without toxicity or side effects to the hematological system and internal organs. Collectively, for the first time, they showed that ciclopirox ethanolamine exerted tumor-suppressor effects in LUAD via TOP2A, suggesting ciclopirox ethanolamine could potentially function as a promising chemotherapeutic for LUAD treatment.[5]
Conclusions
Ciclopirox ethanolamine is the only marketed hydroxypyridone antifungal agent. It is available worldwide in various formulations for the treatment of superficial fungal infections, including tinea pedis, tinea cruris, tinea corporis, cutaneous candidiasis, and tinea versicolor. In addtion, ciclopirox ethanolamine has been considered as an anti-cancer candidate drug recently.
References
[1] Łabędź N, Navarrete-Dechent C, Kubisiak-Rzepczyk H, Bowszyc-Dmochowska M, Pogorzelska-Antkowiak A, Pietkiewicz P. Pityriasis Versicolor-A Narrative Review on the Diagnosis and Management. Life (Basel). 2023;13(10):2097. Published 2023 Oct 22. doi:10.3390/life13102097
[2] Jue SG, Dawson GW, Brogden RN. Ciclopirox olamine 1% cream. A preliminary review of its antimicrobial activity and therapeutic use. Drugs. 1985;29(4):330-341. doi:10.2165/00003495-198529040-00002
[3] Abrams BB, Hänel H, Hoehler T. Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol. 1991;9(4):471-477. doi:10.1016/0738-081x(91)90075-v
[4] Wan X, Xiang J, Fan H, et al. Ciclopirox Olamine Induces Proliferation Inhibition and Protective Autophagy in Hepatocellular Carcinoma. Pharmaceuticals (Basel). 2023;16(1):113. Published 2023 Jan 12. doi:10.3390/ph16010113
[5] Yin J, Che G, Jiang K, et al. Ciclopirox Olamine Exerts Tumor-Suppressor Effects via Topoisomerase II Alpha in Lung Adenocarcinoma. Front Oncol. 2022;12:791916. Published 2022 Feb 18. doi:10.3389/fonc.2022.791916
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPOES is a surface active, non-ionic emulsifying agent. The use of emulsifying or wetting agents in enhancing the growth of mycobacteria is well established.....
May 6,2025SurfactantCiclopirox ethanolamine
41621-49-2You may like
Ciclopirox ethanolamine manufacturers
- Ciclopirox ethanolamine
-
- $0.00 / 1kg
- 2025-12-15
- CAS:41621-49-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Ciclopirox ethanolamine
-

- $0.00 / 1Kg/Bag
- 2025-12-15
- CAS:41621-49-2
- Min. Order: 1KG
- Purity: Aminothanol: 22.2%~23.3% Ciclopirox: 76.0%~78.5%
- Supply Ability: 500KGS
- Ciclopirox olamine
-
- $30.00 / 25mg
- 2025-12-12
- CAS:41621-49-2
- Min. Order:
- Purity: 99.16%
- Supply Ability: 10g