Applications of Dextran Sulfate Sodium Salt
Oct 18,2019
Dextran sulfate sodium salt (DSS) is a polyanionic derivative of dextran produced by esterification of dextran with chlorosulphonic acid. It is a long chain polymer of sulfated glucose and the sulfur content is approximately 17% which corresponds to an average of 1.9 sulfate groups per glucosyl residue of the dextran molecule. DSS is the sodium salt which is a white to off-white powder freely soluble in water and salt solutions to form a stable, clear solution. The high purity and reproducible quality enable its application in pharmaceuticals and biotechnology industry.
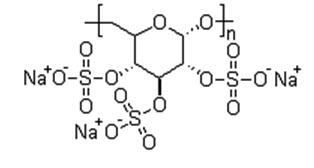
Dextran sulfate sodium salt (DSS) is used as a nucleic acid hybridization accelerant. The polyanionic character of Dextran sulfate helps to approximate nucleic acid strands, increasing their effective concentration to each other and promoting hybridization. It also demonstrates a sequestering interaction with proteins, presumably through hydrogen-bonding between the sulfate groups and amine groups of the protein that generates an insoluble complex. The ability of the polymer to precipitate fibrinogen and low-density lipoproteins from plasma solutions has been used for analysis and for anticoagulant applications. Dextran sulfate sodium salt (DSS) has been proven to have versatile value in Biomedical/clinical research. Dextran sulfate sodium salt (DSS) can be used to study Colitis (Inflammatory Bowel disease) as well as an antiviral as agent. Oral administration of dextran sulfate induces colonic inflammation in a mouse model of inflammatory bowel disease (IBD). Acute, chronic, and relapsing models of IBD can be induced by varying the concentration and administration frequency of dextran sulfate.
Dextran sulfate sodium salt (DSS) shows immunologically relevant activities: enhancement and suppression of humoral immunity, polyclonal activation of B-lymphocytes, stimulating even immature B cells, changes in thymocyte reactivity to lectins, enhancement/suppression of cell-mediated immune responses. Precipitates LDL and VLDL lipoproteins - in the presence of magnesium ions, dextran sulfate precipitates low-density lipoproteins from human serum leaving the high-density lipoproteins in the supernatant. Removal of lipoproteins by dextran sulfate precipitation may be useful in the purification of other materials such as beta 2 glycoprotein.
Dextran sulfate sodium salt (DSS) has been widely used for multiple applications in clinical, molecular biology, biomedical and possibly for cosmetic applications. It has been tested as a potential substitute for heparin in anticoagulant therapy. Ingelman, Walton and Ricketts explored the anticoagulant properties of a wide range of dextran sulfates (Mw 7000 to 458000) and established the lowest molecular weight products displayed the highest anticoagulant properties. However, at best this only represented 15% of heparin's activity. With the introduction of low-dose heparin therapy for thrombosis prophylaxis in the 70's, interest in low molecular weight polyanions has been rekindled and reports on interactions with individual enzyme/inhibitor systems in the coagulation cascade appeared. Dextran sulfate immobilized on cellulose has been found to remove LDL cholesterol preferentially during plasmaphoresis. On the other hand, its wide spectrum of biological properties has attracted the attention of many scientists. It has been used to accelerate hybridization of DNA probes to immobilized nucleic acids[1]. Additionally, it can increase hybridization rate of nucleic acids and show activity as an adjuvant. Furthermore, it shows immunologically relevant activities[2], such as, enhancement and suppression of humoral immunity [3, 4], changes in thymocyte reactivity to lectins [1], inhibition of blood coagulation and platelet aggregation, enhancement of blood fibrinolytic activity [5], and so on.
The toxicity of Dextran sulfate sodium salt (DSS) had been recognized at an early stage. Nevertheless, a low molecule weight fraction with Mw 7000 and S, 16% was considered to be qualitatively similar to heparin. Preliminary clinical trials were unfortunately discouraging and revealed severe adverse reactions, notably, stiff and painful joints, skin eruption, loss of hair and gastro-intestinal symptoms. In chronic toxicity studies in animals, retardation in weight gain and osteoporosis with spontaneous fractures were observed. It should be noted that the doses in the clinical trials corresponded to 1.3 g/day, which is approximately tenfold that used in current heparin therapy.
Recently, attention has been focused on the toxicity of orally administered substance. Supplements of 0.25% and 0.5% for 82 weeks in rats did not increase the incidence of infection or tumours. Higher doses (1~10%) resulted in mortality and tumours. Apart from dosage, the toxicity is also dependent on Mw. Adsorption studies in the gut using a wide range of dextran sulfates (3000~200000) suggest only a small proportion of the dose is absorbed. Intravenous administration has also been investigated. The inhibition of metastasis by dextran sulfate (Mw 6~7 х 103 , DS approx. 2) has been tested clinically but the 5 year survival rate in lung cancer was not improved. Tests for mutagenicity and cytogenicity of low Mw dextran sulfate proved negative.
Reference
[1] Blitstein-Willinger, E., Schutz, G. and Diamanststein, T., "Changes in thymocyte reactivity to lectins by B-cell mitogens of the type of sulphated polyanions." Immunology, v. 30, 529- 533 (1976).
[2] Bradfield, J.W.B., Souhami, R.L., Addison, J.E., "The mechanism of the adjuvant action of dextran sulphate." Immunology, v. 26, 383-386 (1974).
[3] Diamantstein, T., et al., "Stimulation of humoral antibody formation by polyanions. II. The influence of sulphate esters of polymers on the immune response on mice." Eur. J. Immunol., v. 1, 340-343 (1971).
[4] Diamantstein, T., et al., "Suppression of the primary immune response in vivo to sheep red blood cells by B-cell mitogens." Immunology, v 30, 401-405 (1976).
[5] Nakajima, K., et al., "Effects of dextran sulphate on blood coagulation and fibrinolysis in spontaneously hypertensive rats." Adv. Inflamm. Res., v.1, 331-337 (1979).
- Related articles
- Related Qustion
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic o....
Oct 18,2019Organic ChemistryCreatine is a chemical that is found in the body. It is found mostly in muscles but also in the brain. It is also found in foods such as red meat and seafood. Creatine can also be made in the laboratory.....
Oct 18,2019Biochemical EngineeringDextran Sulfate Sodium Salt
9011-18-1You may like
Dextran Sulfate Sodium Salt manufacturers
- Dextran Sulfate Sodium Salt
-

- $0.00 / 1KG
- 2025-12-18
- CAS:9011-18-1
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Dextran sulfate sodium salt
-

- $0.00 / 1kg
- 2025-12-18
- CAS:9011-18-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- Dextran Sulfate Sodium Salt
-

- 2025-12-17
- CAS:9011-18-1
- Min. Order:
- Purity: 0.99
- Supply Ability:






