Argatroban: pharmacology and safety
Jul 7,2025
Introduction
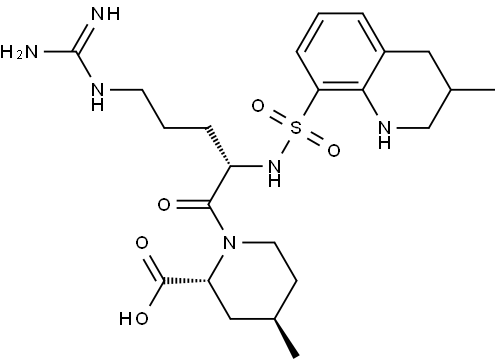
Argatroban (Figure 1) is a small-molecule, synthetic, direct thrombin inhibitor, which competitively binds to the active site of thrombin. It binds rapidly and reversibly to thrombin, thereby inhibiting fibrin formation, the activation of coagulation factors V,VIII and XIII, and the natural anticoagulant proteinC. The use of argatroban does not lead to the formation of antiplatelet antibodies or the generation of other antibodies that bind argatroban and it has no structural similarity to heparin.The American College of Cardiologists (ACC) recommend using bivalirudin or argatroban in patients who have had, or at risk for, heparin induced thrombocytopenia (HIT) and are undergoing percutaneous coronary intervention. Argatroban is a non-heparin anticoagulant shown to both normalize platelet count in patients with HIT and prevent the formation of thrombi. Parental anticoagulants must be stopped and a baseline activated partial thromboplastin time must be obtained prior to administering argatroban.[1]

Chemistry
Argatroban consists of two isomers. The ratio of R to S isomer in argatroban is approximately 65:35. The R-and S-isomers have been shown to exhibit different anticoagulant and antithrombotic potencies.
Pharmacokinetics & metabolism
Argatroban is typically administered as an intravenous continuous infusion with or without a bolus injection. The time to steady state drug levels in individuals with normal clearance is predictable and has been reported to be 1-3 h. The steady-state drug level increases in proportion to dose (at least up to infusions of 40 µg/kg/min). Upon cessation of infusion, plasma drug levels decay with a half-life of 39-51 min. A nomogram has been developed to help rapidly achieve and maintain doses in the therapeutic range and to guide dosing adjustments when required. The nomogram assumes an initial dose of 2 µg/kg/min. If at 3 h, the activated partial thrombo plastin time (aPTT) is subtherapeutic(<44s), the infusion is increased by 0.5 µg/kg/min and the aPTT is again measured 3h later. If the aPTT is between 91 and 121s,the infusion is decreased by 0.5 µg/kg/min and the aPTT is again measured 3 h later. For aPTTs of more than 121 s, the infusion is held before resuming at 50% of the original rate.
Argatroban can be differentiated from the other parenteral DTIs by its hepatic (not renal) clearance. All of the identified metabolites of argatroban are produced by metabolism of the 3-methyl-1,2,3,4-tetrahydroquinoline ring of the molecule by hepatic CYP3A4/5 oxidases. M-1, the major metabolite of argatroban, exhibits a weaker anticoagulant effect than argatroban. The circulating amount of M-1 is less than 20% of that of intact argatroban. The majority of argatroban is excreted via the feces,likely via biliary excretion.[2]
Pharmacodynamic Profile[1]
The pharmacological properties of argatroban(e.g. selective and reversible inhibition of the thrombin catalytic site, rapid onset and short duration of action) offer the potential for significant antithrombotic efficacy and rapid restoration of haemostasis after discontinuation of the drug.
Thrombin Binding and Inhibition
Argatroban binds rapidly at a diffusion-controlled rate to the catalytic site domain of thrombin. Unlike hirudin and bivalirudin the interaction is fully reversible. It has an inhibition constant of 3.9×10-8mol/L for human thrombin. Argatroban is an effective inhibitor of thrombin bound to fibrin clots as well as thrombin free in solution. In contrast, heparin and hirudin are less potent in inhibiting bound versus soluble thrombin. In vitro the plasma argatroban concentrations required to inhibit by 50% (IC50) the activity of soluble, fibrin-bound and clot-bound thrombin were, respectively, 1.1, 2.8 and 2.4 µmol/L. Heparin and hirudin each showed over 1000-fold decreases in inhibitory potency for clot-bound versus soluble thrombin.
Argatroban is an effective inhibitor of thrombin bound to aged fibrin clots and of thrombin bound to fibrin clots already treated with streptokinase or alteplase. Blood clots formed in vitro from the blood of healthy adults were aged and some were treated with streptokinase or alteplase. In a clot permeation model, the IC50 of argatroban for clots aged 3 hours was 2.7µmol/L, which was not significantly different from those aged 6 hours (3.4µmol/L). Argatroban inhibited approximately 85%of the thrombin activity present in these aged fibrin clots. In clots treated with streptokinase or alteplase, a saturating argatroban concentration inhibited thrombin by approximately 70%.
Anticoagulant Effects
In several animal models the antithrombotic effect (e.g. reduction in mean thrombus weight, increase in duration of postlesion vessel patency) of argatroban was similar to that of heparin; however,argatroban was associated with less systemic anticoagulation of haemorrhagic potential [e.g. increase in activated partial thromboplastin time (aPTT) or bleeding time. The effect half-life for argatroban, given by continuous infusion at 4 escalating dose levels of 1.25,2.5, 5 or 10 µg/kg/min, with or without a bolusloading dose of 250 µg/kg, was approximately 18 to 40 minutes and was similar for both aPTT and activated clotting time (ACT). In contrast, the effect half-life for heparin (0.15, 0.20, 0.25 or 0.30U/kg/min, with or without a bolus-loading dose of 125U/kg), was somewhat longer than argatroban and was different for aPTT and ACT. When argatroban was administered by continuous infusion alone in healthy volunteers, steady-state anticoagulant effects were achieved within 1 to 3hours.
Effects on Vascular Smooth Muscle
In canine coronary arteries the concentration of argatroban required to inhibit the vasoconstrictive effects of thrombin by 50% (ED50) was 0.3 µmol/L. In the presence of an intact endothelium, argatroban did not inhibit relaxation dependent on the endothelium-derived relaxing factor induced by trypsin, acetylcholine or the calcium ionophore A23187. In segments stripped of endothelium, argatroban partially inhibited the vasoconstrictive effects of thrombin.
Safety & tolerability
As with all anticoagulant drugs, the major safety issue relates to the incidence of hemorrhage. A retrospective analysis of risk factors for major bleeding in patients with HIT who were treated with argatroban has been reported. In this analysis, the presence of HIT-related thrombus at the initiation of treatment, pulmonary impairment and an aPTT of over 100 s were associated with an increased incidence of major bleeding. Argatroban administration requires careful monitoring inpatients with heart failure, multiple organ failure and those with hepatic failure to avoid unwanted bleeding, as discussed above.Characteristics of argatroban that have been discussed above,which give it a wider safety window not shared by other parenteral DTIs, include its nonimmunogenic nature and nonrenal elimination. General tolerability to argatroban is seen with lack of effect to age, gender, obesity and drug interactions.[2]
References
[1] McKeage K, Plosker GL. Argatroban. Drugs. 2001;61(4):515-524. doi:10.2165/00003495-200161040-00005
[2] Jeske WP, Fareed J, Hoppensteadt DA, Lewis B, Walenga JM. Pharmacology of argatroban. Expert Rev Hematol. 2010;3(5):527-539. doi:10.1586/ehm.10.53
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThioglycolic acid is a chemical compound commonly used in cosmetics, particularly in hair removal products. Its primary function is to break down the protein structure of hair, making it more pliable and easier to remove.....
Jul 7,2025Chemical ReagentsArgatroban
74863-84-6You may like
- Argatroban USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:74863-84-6
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Argatroban
-
- $33.00 / 5mg
- 2025-11-10
- CAS:74863-84-6
- Min. Order:
- Purity: 99.97%
- Supply Ability: 10g
- Argatroban
-
- $33.00 / 5mg
- 2025-11-04
- CAS:74863-84-6
- Min. Order:
- Purity: 99.97%
- Supply Ability: 10g