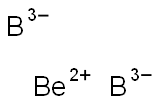Beryllium Diboride: Crystal Structure
Apr 3,2024
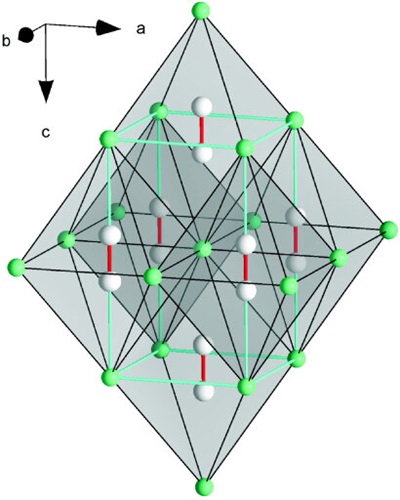
It was shown that Beryllium Diboride has four stable crystal structures, cubic (space group , NO.216), orthorhombic (space group Pnma, NO.62), orthorhombic (space group Pbam, NO.55 ) and hexagonal (space group P63/mmc, NO.194). A systematic structure search of BeB2 led to the discovery of a new stable phase with the space group NO.63. The conventional unit cell of the new Cmcm phase contains a total of 12 atoms, with Be and B atoms located at two unequal Wyckoff positions, 4c and 8f, respectively.

The schematic crystal structure of the Cmcm phase is shown in Figures 1, 2 and 3, where yellow and blue spheres represent Be and B atoms, respectively. Figure 1 shows the unit cell, while Figure 2 shows the super-cell of the same crystal structure. Figure 2(b) is perpendicular to the x-axis. There are two parallel columns formed by boron-centered and beryllium-centered coordination polyhedra that are marked by green and blue colors, respectively. Each boron atom is surrounded by four beryllium atoms that form a distorted tetrahedral configuration. Eight boron atoms embrace a beryllium atom, forming a complex polyhedron composed of 3 rectangles and 6 triangles. Along the z-axis, the structure can be viewed as several layers, as shown in Figure 2 (b) and (c). Six boron atoms connect to each other in a hexagonal ring, where 4 atoms are coplanar (denoted as B and B*) and the other 2 atoms are out of this plane (denoted as A and A*). Be atoms form another layer (denoted as C) between the B and B* layers along the z-axis. The upper image of Figure 2 (a) is taken from the A, B, C, B* and A* layers, whereas the lower image is composed of the B*, A*, C*, A and B layers. The distance between two A layers is 5.120 Å. The configurations of boron-centered and beryllium-centered coordination polyhedra are displayed in Figure 3. The distances of the three types of Be-B bonds are 1.923 Å, 2.021 Å and 2.105 Å, respectively, as shown in Figure 3 (a). The Boron-Boron bond lengths are 1.763 Å (the blue bond) and 1.657 Å (the green bond), as shown in Figure 3 (b).


- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringLanthanum carbide (LaC2) is a chemical compound. It is being studied about the manufacture of certain types of superconductors and nanotubes.....
Apr 7,2024Inorganic chemistryberyllium diboride
12536-51-5You may like