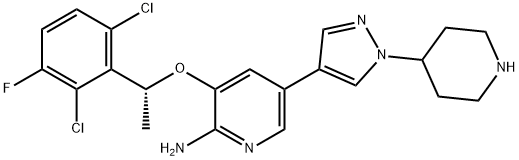
Crizotinib: clinical activity, relapses and mechanisms of resistance
Jan 16,2024
General Description
Crizotinib, an ATP competitive inhibitor of cMET, ALK, and ROS1 kinases, has demonstrated significant clinical activity in the treatment of ALK+ NSCLC. Initial phase I and II trials showed a high objective response rate (ORR) of 61% and 50% respectively. Subsequent phase III studies confirmed the efficacy of crizotinib, with improved progression-free survival (PFS) and ORR compared to chemotherapy in both the second-line and upfront settings. Despite its initial positive responses, clinical relapses are common, often occurring within the first year of treatment. Relapses can manifest as CNS-only involvement or oligoprogression at extracranial sites. Mechanisms of resistance include secondary mutations in the ALK kinase domain, ALK copy number alterations, bypass tracks, paracrine factors, and EMT. Understanding these mechanisms is crucial for developing strategies to overcome or prevent resistance and improve treatment outcomes.

Figure 1. Capsules of crizotinib
Clinical activity
Crizotinib is an oral small molecule ATP competitive inhibitor that was originally developed as a cMET kinase inhibitor. However, it was later discovered to also inhibit ALK and ROS1 kinases. This led to the inclusion of patients with ALK+ NSCLC in the initial phase I trial of crizotinib, which aimed to investigate its efficacy in various types of advanced cancer. The results from this trial showed promising clinical activity, with an objective response rate (ORR) of 61% in the first 119 assessable patients. These findings were further supported by the phase II trial, where an ORR of 50% was observed in the first 136 patients treated with crizotinib. As a result, the FDA granted accelerated approval for crizotinib on August 26, 2011. Two subsequent phase III studies were conducted to evaluate the efficacy of crizotinib. In the first study, crizotinib was compared to single-agent chemotherapy in the second-line setting. It significantly improved progression-free survival (PFS) from 3.0 to 7.7 months, with an ORR of 65% compared to 20% with chemotherapy. In the second study, crizotinib was compared to platinum-based combination chemotherapy in the upfront setting. It significantly improved PFS from 7.0 to 10.9 months, with an ORR of 74% compared to 45% with chemotherapy. Both phase III studies demonstrated that crizotinib was well-tolerated and resulted in a greater improvement in quality of life compared to chemotherapy. Based on the positive data from PROFILE 1007, crizotinib received full approval from the FDA on November 20, 2013. It was initially approved by the EMA as a second-line therapy and later received approval for use in the first-line setting on November 24, 2015. Crizotinib is also approved in many other countries for the treatment of advanced, ALK+ NSCLC patients. 1
Clinical relapses
Clinical relapses of crizotinib commonly occur despite initial positive responses. Most relapses happen within the first year of treatment, but some patients may experience prolonged responses lasting over 6 years. In the majority of cases, disease progression after crizotinib treatment involves multiple sites. However, a smaller proportion of patients may have oligoprogression, which is limited progression to a few metastatic sites. One pattern of relapse is central nervous system (CNS) only relapses. Brain metastases are frequently present at diagnosis and during disease progression on crizotinib. Despite improved disease control with crizotinib compared to chemotherapy, CNS progression still occurs. This is attributed to the pharmacokinetic limitations of crizotinib in crossing the blood-brain barrier. However, resuming crizotinib after local treatments for brain metastases has shown promise in controlling extracranial disease. Another pattern is oligoprogression at extracranial sites. Some patients may experience progression limited to a few sites. Interruption of crizotinib for local ablative therapy has been explored as a strategy to eradicate resistant clones and capitalize on ALK dependence at responding sites. Limited studies have shown extended progression-free survival with this approach. In conclusion, clinical relapses of crizotinib in ALK+ NSCLC can manifest as CNS-only relapses or oligoprogression at extracranial sites. Strategies such as resuming crizotinib after local ablative therapy have shown promise in managing these unique patterns of treatment failure. 2
Mechanisms of resistance
Crizotinib initially elicits a 91% disease control rate. However, the mechanisms behind intrinsic resistance are poorly understood, while acquired resistance commonly occurs after an average treatment duration of 11.3 months. Acquired resistance mechanisms include secondary mutations in the ALK kinase domain, ALK copy number alterations, bypass tracks, paracrine factors, and epithelial–mesenchymal transition (EMT). Secondary resistance mutations in the ALK kinase domain have been identified in 22-36% of patients. These mutations, such as L1196M and C1156Y, impact crizotinib binding or increase ALK catalytic activity. Additionally, ALK copy number gain has been recognized as a resistance mechanism. Approximately two-thirds of resistant patients do not exhibit identifiable secondary mutations or copy number alterations. In these cases, aberrant activation of alternate kinases, such as EGFR or KIT, bypasses ALK signaling. Paracrine factors, including EGFR ligands and neuregulin, contribute to resistance by activating downstream signaling cascades independent of ALK. Moreover, EMT, which is associated with enhanced migratory and invasive capacity, may also contribute to resistance. In conclusion, understanding the diverse mechanisms of resistance to crizotinib is crucial for developing effective strategies to overcome or prevent resistance and improve treatment outcomes for patients with ALK+ NSCLC. 3
Reference
1. Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27 Suppl 3(Suppl 3):iii42-iii50.
2. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011-1019.
3. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer [published correction appears in N Engl J Med. 2015 Oct 15;373(16):1582]. N Engl J Med. 2014;371(23):2167-2177.
- Related articles
- Related Qustion
- Adverse reactions of Crizotinib in the treatment of Inflammatory myofibroblastic tumour (IMT) Nov 3, 2023
The most common adverse reactions (≥35%) in pediatric patients were vomiting, nausea, diarrhea, abdominal pain, rash, vision disorder, upper respiratory tract infection, cough, pyrexia, musculoskeletal pain, fatigue, edema, constipation, an
- Crizotinib: From Discovery to Front-line Treatment Apr 7, 2023
Crizotinib (XALKORI) is a potent and selective mesenchymal epithelial factor/anaplastic lymphoma kinase (c-Met/ALK) inhibitor.
- The activity of Crizotinib in lung cancer Oct 31, 2019
Crizotinib is an anti-cancer drug acting as an ALK (anaplastic lymphoma kinase) and ROS1 (c-ros oncogene 1) inhibitor.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringRemogliflozin etabonate (RE) is the prodrug of remogliflozin, a selective sodium?glucose cotransporter subtype 2 (SGLT2) inhibitor discovered by Kissei Pharmaceutical.....
Jan 16,2024APICrizotinib
877399-52-5You may like
- Crizotinib
-
- 2025-12-05
- CAS:877399-52-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Crizotinib
-

- $44.00 / 10mg
- 2025-12-05
- CAS:877399-52-5
- Min. Order:
- Purity: 99.76%
- Supply Ability: 10g
- Crizotinib
-

- $5.00/ KG
- 2025-12-05
- CAS:877399-52-5
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS