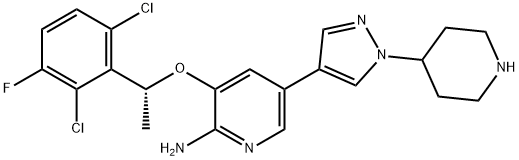
Adverse reactions of Crizotinib in the treatment of Inflammatory myofibroblastic tumour (IMT)
Nov 3,2023
On July 14, 2022, the Food and Drug Administration approved crizotinib (Xalkori, Pfizer Inc.) for adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory inflammatory anaplastic lymphoma kinase (ALK)-positive myofibroblastic tumors (IMT). And in clinical outcomes, the most common adverse reactions (≥35%) in pediatric patients were vomiting, nausea, diarrhea, abdominal pain, rash, vision disorder, upper respiratory tract infection, cough, pyrexia, musculoskeletal pain, fatigue, edema, constipation, and headache. The most frequent adverse reactions (≥35%) in adult patients were vision disorders, nausea, and edema.The recommended crizotinib dose in adult patients is 250 mg orally twice daily until disease progression or unacceptable toxicity. The recommended pediatric dose is 280 mg/m2 orally twice daily until disease progression or unacceptable toxicity.
- Related articles
- Related Qustion
- Crizotinib: clinical activity, relapses and mechanisms of resistance Jan 16, 2024
Crizotinib shows clinical activity in ALK+ NSCLC. Relapses occur within a year, CNS-only or oligoprogression. Resistance mechanisms include ALK mutations, copy number alterations and bypass tracks.
- Crizotinib: From Discovery to Front-line Treatment Apr 7, 2023
Crizotinib (XALKORI) is a potent and selective mesenchymal epithelial factor/anaplastic lymphoma kinase (c-Met/ALK) inhibitor.
- The activity of Crizotinib in lung cancer Oct 31, 2019
Crizotinib is an anti-cancer drug acting as an ALK (anaplastic lymphoma kinase) and ROS1 (c-ros oncogene 1) inhibitor.
N-Glycyl-L-tyrosine demonstrates potent antioxidant and anti-inflammatory properties, making it a promising therapeutic agent with clinical applications.....
Nov 3,2023APITetrabutyl titanate is commonly used as a catalyst. It is able to improve the adhesion of coatings, rubber, and plastics to metal surfaces.....
Nov 6,2023Organic reagentsCrizotinib
877399-52-5You may like
- Crizotinib
-
- 2025-12-14
- CAS:877399-52-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Crizotinib
-

- $5.00/ KG
- 2025-12-12
- CAS:877399-52-5
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Crizotinib
-

- $44.00 / 10mg
- 2025-12-10
- CAS:877399-52-5
- Min. Order:
- Purity: 99.76%
- Supply Ability: 10g