Is CH2Cl2 Polar or Nonpolar?
Dec 20,2023
Dichloromethane CH2Cl2 is a polar molecule. It(CH2Cl2) consists of a central carbon (C) atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen (H) atoms and two chlorine (Cl) atoms.
The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape.The such arrangement leads to a tetrahedral geometry with a bond angle of 109.5°.
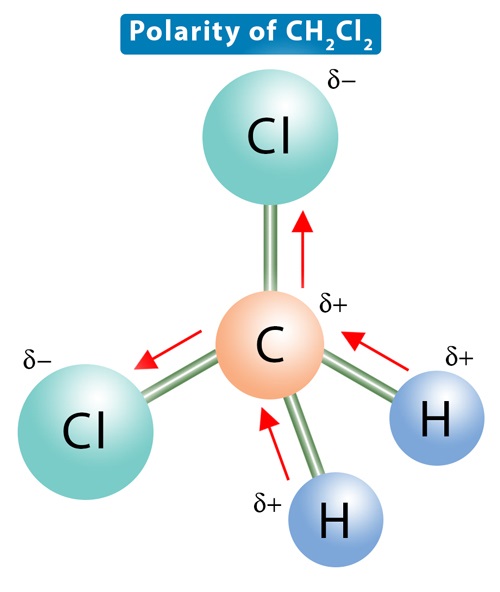
The electronegativity of carbon is 2.55, that of chlorine is 3.16, and that of hydrogen is 2.2. An electronegativity difference of 0.61 units exists between a carbon and a chlorine atom in the C-Cl bond in the CH2Cl2 molecule. Therefore, the C-Cl bonds are more polar than the C-H bonds, so there is some net residual polarity. The bond dipoles are arranged asymmetrically. There is no way to arrange the molecule such that the dipole moments cancel out. As a result, there will be a net dipole moment, making dichloromethane a polar molecule.
- Related articles
- Related Qustion
- Toxicity of Dichloromethane Jan 18, 2022
Dichloromethane (DCM; mol. wt. 93.328) was first prepared in 1840 by mixing chloromethane and chlorine and exposed to sunshine. It has been used as a versatile solvent to dissolve various organic compounds in many chemical processes since W
- Toxicity hazards of Dichloromethane Nov 18, 2021
Dichloromethane can be used as a solvent, dental local anesthetic, refrigerant and fire extinguishing agent. In addition to being used in organic synthesis, dichloromethane is often used as a non-flammable solvent for flammable materials.
- Uses of Dichloromethane Nov 20, 2019
Dichloromethane is a geminal organic chemical. It is also known to the scientists under the names methylene chloride or methylene dichloride. The substance may also be called refrigerant-30 freon-30, R-30, DCM, UN 1593, solmethine, narkotil
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGlucose is a polar molecule due to its multiple hydroxyl groups, which create polar bonds. The polarity of glucose plays a essential role in biological processes such as energy metabolism.....
Dec 20,2023Biochemical EngineeringDichloromethane
75-09-2You may like
- DCM
-

- $10.00 / 1KG
- 2025-12-11
- CAS:75-09-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Dichloromethane
-
- $0.00 / 200kg
- 2025-11-19
- CAS:75-09-2
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Dichloromethane
-

- $450.00 / 10ton
- 2025-09-26
- CAS:75-09-2
- Min. Order: 50ton
- Purity: 99
- Supply Ability: 500 ton






