Domperidone: A Benzimidazole Derivative's Efficacy and Pharmacokinetics in Treating Gastrointestinal Disorders
Feb 6,2024
General Description
Domperidone, a benzimidazole derivative known for its peripheral D2 receptor blockade, primarily affects the gastrointestinal tract due to its limited blood-brain barrier penetration. It shows variable bioavailability, significantly reduced upon oral intake due to first-pass metabolism. While its efficacy in treating nausea and vomiting, particularly in acute gastroenteritis in children, is debated and generally considered less effective than ondansetron, domperidone has also been explored for Gastro-Oesophageal Reflux Disease. Despite some positive outcomes, especially when combined with proton pump inhibitors, robust evidence supporting its use in children for GORD is lacking, leading to hesitance in its endorsement by major health guidelines due to efficacy and safety concerns.

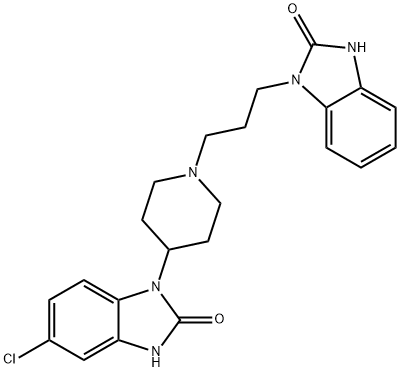
Figure 1. Domperidone
Pharmacokinetics
Domperidone, a benzimidazole derivative, exhibits its pharmacological effects primarily through peripheral dopamine-2 (D2) receptor blockade, particularly in the gastrointestinal tract, due to its limited ability to cross the blood-brain barrier. Upon oral administration, domperidone is rapidly absorbed, with peak plasma levels achieved within 30 to 60 minutes, and demonstrates a high plasma protein binding rate of up to 93%. Its bioavailability significantly differs based on the route of administration: approximately 90% following intramuscular injection, but drastically reduced to 13-17% after oral intake, attributed to extensive first-pass hepatic metabolism which converts it into inactive metabolites. The reduction in oral bioavailability is further exacerbated by the use of antacid drugs that increase gastric pH. Domperidone undergoes metabolism predominantly through aromatic hydroxylation and oxidative N-dealkylation via the cytochrome P450 isoenzyme 3A4 (CYP3A4) present in the liver and small intestine. Despite its widespread distribution in body tissues, its central nervous system penetration is minimal. The presence of CYP3A4 inhibitors can elevate free plasma concentrations of domperidone up to threefold. It crosses the placenta and is excreted in breast milk only in small amounts, reflecting its extensive first-pass metabolism. 1
Indications and efficacy
Nausea and vomiting
Domperidone has been explored as a treatment for nausea and vomiting, particularly in cases of acute gastroenteritis in children, a condition frequently associated with rotavirus infection. Its effectiveness, however, remains under debate when compared to other antiemetics like ondansetron. Research indicates mixed results: one study showed domperidone was less effective than ondansetron in stopping vomiting at 24 hours, yet another study indicated no significant difference between the two after 6 hours, although ondansetron proved superior at the 24-hour mark. Additionally, ondansetron demonstrated advantages over domperidone in enhancing tolerance for oral rehydration therapy, reducing the need for intravenous fluids, and decreasing hospital stays. A specific study highlighted that a low dose of domperidone did not significantly reduce vomiting episodes compared to placebo in children under 12 years. Furthermore, while rectal administration of domperidone alongside oral rehydration showed some effectiveness after 72 hours, it wasn't superior in the early stages of treatment. Conclusively, current evidence supports ondansetron as the preferred option for treating vomiting in acute gastroenteritis over domperidone, with a meta-analysis affirming ondansetron's role in effectively managing symptoms and reducing hospital intervention needs. 2
Gastro-oesophageal reflux disease
Domperidone's role in treating Gastro-Oesophageal Reflux Disease has been explored, given its ability to increase lower oesophageal sphincter pressure, potentially benefiting patients inadequately responding to proton pump inhibitors. Recent analyses suggest that combining domperidone with PPIs may enhance treatment outcomes for both adults and children, improving symptoms like heartburn and reflux frequency without increasing side effects. However, evidence regarding domperidone's effectiveness in infants and children remains conflicting, with some studies indicating positive outcomes, while others show no significant improvement compared to placebo. Notably, a Cochrane review emphasizes the lack of robust randomized controlled trial evidence supporting domperidone's use in children. Despite some studies suggesting potential benefits, such as reduced symptom severity and decreased reflux episodes, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the National Institute of Health Excellence guidelines do not endorse domperidone for treating GORD in infants and children, citing uncertainties about its efficacy and risk profile. 2
Reference
1. Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS. Domperidone. A review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs. 1982;24(5):360-400.
2. Puoti MG, Assa A, Benninga M, et al. Drugs in Focus: Domperidone. J Pediatr Gastroenterol Nutr. 2023;77(2):e13-e22.
- Related articles
- Related Qustion
- Domperidone: mechanism of action and clinical applications in gastroenterology Aug 1, 2023
Domperidone blocks dopamine receptors, helping to relieve gastrointestinal symptoms like nausea and vomiting. It is used for GERD, dyspepsia, and postoperative nausea/vomiting.
- What is Domperidone?Pharmacodynamics,Uses,Side effects,Precautions Sep 10, 2020
Domperidone is a specific blocker of dopamine receptors. It speeds gastrointestinal peristalsis, causes prolactin release, and is used as antiemetic and tool in the study of dopaminergic mechanisms.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringQuercetin is the most common flavonoid, existing primarily in its glycoside form as quercitrin (3-rhamnoside).....
Feb 6,2024Natural ProductsDomperidone
57808-66-9You may like
- Domperidone
-
- $54.00 / 100mg
- 2025-12-05
- CAS:57808-66-9
- Min. Order:
- Purity: 99.82%
- Supply Ability: 10g
- Domperidone
-
- $54.00 / 100mg
- 2025-12-05
- CAS:57808-66-9
- Min. Order:
- Purity: 99.82%
- Supply Ability: 10g
- Domperidone
-

- $0.00 / 1kg
- 2025-12-05
- CAS:57808-66-9
- Min. Order: 1kg
- Purity: 99%-101%; EP
- Supply Ability: 100kgs