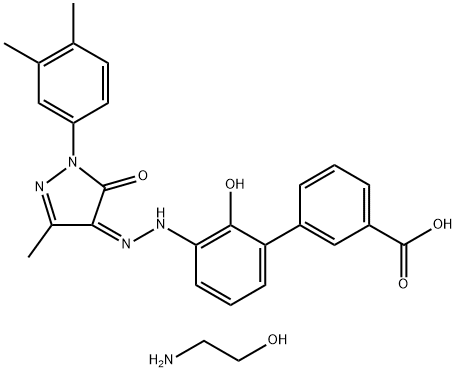
Eltrombopag Olamine in Thrombocytopenia Treatment
Aug 26,2025
Eltrombopag olamine is approved to treat thrombocytopenia (low platelet levels). It is used in adults and in children aged 1 year or older with chronic immune thrombocytopenia (ITP). ITP is a condition in which platelets are destroyed by the immune system. Eltrombopag olamine is used in certain patients with ITP who have not gotten better with other treatment. Drugs are often studied to find out if they can help treat or prevent conditions other than the ones they are approved for. This patient information sheet applies only to approved uses of the drug. However, much of the information may also apply to unapproved uses that are being studied. Eltrombopag is not recommended for use in children and adolescents below the age of 18 years due to insufficient data on safety and efficacy. Even though no increased incidence of thromboembolic events, myelofibrosis, or irreversible hepatic damage was reported in follow up over 4 years, patients on eltrombopag should still be monitored closely for such adverse events.

Bioequivalence and Food Effect Assessment of Eltrombopag Olamine Tablets
Eltrombopag is the pioneering oral, small-molecule, nonpeptide thrombopoietin receptor agonist (TPO-RA) that selectively interacts with the transmembrane domain of the TPO receptor. This interaction triggers megakaryopoiesis and platelet production via the activation of the receptor. The pharmacokinetics of eltrombopag olamine tablets is characterized by rapid absorption, reaching maximum plasma concentration (Cmax) within 2-6 hours. Its binding affinity to human plasma proteins, particularly albumin, is exceedingly high (>99.9%). Acknowledging racial disparities, it is imperative to comprehend the clinical pharmacokinetic profile of eltrombopag across diverse populations. Currently, there is a dearth of information regarding the clinical pharmacokinetics and safety of eltrombopag in Chinese individuals, under both fasting and fed conditions. Therefore, this study aimed to investigate the pharmacokinetic profile and safety of generic and reference eltrombopag olamine tablets in healthy Chinese volunteers. The findings of this research will support the marketing approval of the newly developed generic formulation in China.[1]
Given that both fasting and fed conditions are recommended by the US Food and Drug Administration's draft guidance on eltrombopag olamine and the guiding principles of the National Medical Products Administration of China, an open, randomized, single-dose, 2-period, crossover study was devised to assess both of these conditions. To comprehensively examine the food effect of the drug, the subjects ingested the eltrombopag olamine tablets precisely 30 minutes after a meal. Although this meal was classified as low-calcium based on its composition, it contained relatively high levels of other mineral components, particularly magnesium, with a total content exceeding 90 mg. Prior studies have indicated that bioavailability is not significantly altered when administered with a low-calcium and high-fat diet. However, a significant food effect was observed in the fasting and fed clinical trials, wherein administration with food in the fed state led to a net reduction in systemic availability, based on AUC0-∞, of approximately 40%. Therefore, it is recommended that eltrombopag olamine tablets should be taken on an empty stomach or 1 hour before or 2 hours after a meal, particularly avoiding calcium-rich foods or mineral supplements containing polyvalent cations such as magnesium, iron, and copper.
Efficacy and safety profile of Eltrombopag Olamine
Immune thrombocytopenia (ITP) is an immune-mediated disease characterized by transient or persistent decrease of the platelet count to less than 100 × 109/liter. The term ‘newly diagnosed ITP’ is used to describe all cases at diagnosis. Recently, two thrombopoietin-receptor (TPO-R) agonists, romiplostim (AMG-531, Nplate; Amgen, Thousand Oaks, CA, USA) and Eltrombopag Olamine (Revolade, Promacta; GlaxoSmithKline, Brentford, UK) have been licensed for the treatment of chronic ITP. Unlike native thrombopoietin which binds to the extracellular domain of the thrombopoietin receptor, eltrombopag selectively binds to the trans-membrane domain of the receptor. It stimulates megakaryocytopoiesis through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway. Eltrombopag did not affect agonist-induced platelet aggregation or activation in in vitro and ex vivo studies using platelet samples from healthy volunteers and patients with chronic ITP. Eltrombopag Olamine is an orally administered small-molecule nonpeptide TPO-R agonist. It has been shown to effectively increase platelet counts and reduce bleeding symptoms in patients with chronic ITP with overall response rate of 60–80%. Eltrombopag Olamine is well tolerated and has a good safety profile. It is recommended for splenectomized patients with ITP who are refractory to other treatments.[2]
The recommended starting dose of Eltrombopag Olamine is 50 mg once daily. For patients of East Asian ancestry, eltrombopag should be initiated at a reduced dose of 25 mg once daily. After initiating eltrombopag, if no significant increase in platelet counts is observed after 2–3 weeks of treatment, could the dose may be increased. After achieving a stable platelet count on a specific dose, the dose should be further adjusted to the lowest dose sufficient to maintain a platelet count of around 50 × 109/liter with minimal bleeding symptoms. In most responding patients, platelet counts start to increase after the first week of therapy and peak at the second week. Therefore, Eltrombopag Olamine may be used in the preparation of patients with chronic ITP for elective surgery. Patients can start taking eltrombopag at home 2 weeks before the scheduled surgery, thus avoiding presurgical admission for IVIG infusion, which is common practice. Eltrombopag Olamine should be taken at least 4 h before or after any products such as antacids, dairy products (or other calcium-containing food products), or mineral supplements containing polyvalent cations (e.g. iron, calcium, magnesium, aluminum, selenium, and zinc). Eltrombopag was detected in the plasma of all rat pups for the entire sampling period following administration of medicinal product to the mothers, suggesting that rat pup exposure to Eltrombopag Olamine was likely via lactation.
References
[1]Wang, Jingyan et al. “Bioequivalence and Food Effect Assessment of Eltrombopag Olamine Tablets in Healthy Chinese Subjects: An Open, Randomized, Single-Dose, and Two-Period Crossover Study.” Clinical pharmacology in drug development vol. 13,11 (2024): 1260-1266. doi:10.1002/cpdd.1453
[2]Cheng G. Eltrombopag, a thrombopoietin- receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol. 2012 Jun;3(3):155-64. doi: 10.1177/2040620712442525. PMID: 23556122; PMCID: PMC3573439.
- Related articles
- Related Qustion
- Uses of Eltrombopag Olamine in the Treatment of Thrombocytopenia Mar 25, 2025
The use of eltrombopag has been permitted for ITP patients refractory to first-line drugs or splenectomy for the last 10 years. This article will introduce its mechanism, efficacy and safety.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringEltrombopag Olamine
496775-62-3You may like
Eltrombopag Olamine manufacturers
- Unii-4U07F515lg
-
- 2025-12-12
- CAS:496775-62-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Eltrombopag Olamine
-
- $36.00 / 2mg
- 2025-12-12
- CAS:496775-62-3
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g
- Eltrombopag olamine
-

- $0.00 / 1g
- 2025-12-12
- CAS:496775-62-3
- Min. Order: 1g
- Purity: 98.0%min
- Supply Ability: 10KGS