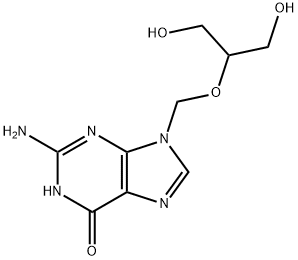
Ganciclovir drug instructions
Aug 26,2020
Background and overview[1][2]
Ganciclovir is a nucleoside antiviral drug, which can competitively inhibit DNA polymerase and is incorporated into the DNA of viruses and host cells, thereby inhibiting DNA synthesis. This product was launched by Syntex in the United States and approved for marketing in 1988. It is the first choice for the treatment of cytomegalovirus infection. In foreign countries, ganciclovir is indicated for the induction and maintenance phase treatment of immunodeficiency patients (including AIDS patients) complicated by cytomegalovirus retinitis, and it can also be used to prevent cytomegalovirus sero-positive AIDS patients in patients receiving organ transplants Prevent the occurrence of cytomegalovirus disease.

The indications specified in the product manual of the generic ganciclovir for injection in China, in addition to the prevention and treatment of immunocompromised patients and giant cell retinitis, are also suitable for hepatitis B, herpes zoster, EB virus infection, etc. . my country's currently marketed dosage forms are mainly powder injections, and a small amount of oral preparations are also available.
Preparation [1]
A preparation method of ganciclovir, which comprises the following steps:
(1) 40kg ganciclovir condensate 7-position isomer, 3.0kg p-toluenesulfonic acid, 400kg solvent-DMF, 77kg 1,3-diacetoxy-2-(acetoxymethoxy) propane, 60kg Diacetylguanine was put into a 1000L reactor, 40kg of acetic anhydride was added, and the temperature was kept at 80~140℃ for 3~5h and stirred, then acetic anhydride was distilled, and the temperature was kept at 120-130℃ for 18 hours, during which distillation 2- Three times of acetic anhydride makes the chemical equilibrium react to the ganciclovir condensate;
(2) After the reaction in the step (1) is completed, the acetic anhydride and the solvent—DMF are evaporated under reduced pressure until there is almost no evaporation to obtain a residue;
(3) Add 500L of ethyl acetate to the residue obtained in the step (2), then raise the temperature to reflux, lower the temperature and cool to 0-5°C, and hold the crystallization time for 1-24 hours;
(4) Centrifuging and spinning the product obtained in the step (3) to obtain crude ganciclovir condensate II;
(5) Add methanol of 10 times the mass of the crude ganciclovir condensate to the crude ganciclovir condensate, heat it up to 100°C and stir for 2h, then cool to 60°C and filter press to obtain filter cake three- The 7-position isomer of ganciclovir condensate and filtrate 3 continue to evaporate methanol and then cool and crystallize to obtain crude ganciclovir condensate 3;
(6) Add 5%-10% sodium hydroxide aqueous solution to the crude ganciclovir condensate III, heat up to 80°C, and hydrolyze for 2h;
(7) Adjust the pH of the hydrolyzed solution in step (6) to neutral, add activated carbon to heat up, then dissolve, decolorize, filter, crystallize, centrifuge, and dry to obtain crude ganciclovir;
(8) Put the crude ganciclovir and the mixed solvent of DMSO and DMF into the reaction kettle, raise the temperature to 100°C and stir for 2h, then cool to 60°C and filter with pressure to obtain the filter cake-ganciclovir 7 Position isomer and filtrate 1--9 ganciclovir, the amount of the mixed solvent of DMSO and DMF is 10 times the amount of crude ganciclovir;
(9) Re-add filter cake 1 and filter cake 3 to the condensation reaction in step (1);
(10) Once the filtrate is continuously cooled to 0°C, kept for 5 hours, centrifuged to obtain pure ganciclovir.
Pharmacological effects [2]
1. Valganciclovir is the L-valyl ester (prodrug) of ganciclovir, which is quickly converted into ganciclovir by esterases in the small intestine and liver after oral administration. Ganciclovir is a synthetic 2'-deoxyguanosine analogue, which can inhibit the replication of herpes virus in vitro and in vivo. Sensitive human viruses include human cytomegalovirus (HCMV), herpes simplex virus-1 and herpes simplex virus-2 (HSV-1, HSV-2), human herpesvirus-6, 7, 8 (HHV-6, 7, 8) Epstein-Barr virus, varicella-zoster virus (VZV) and hepatitis B virus. 2. In cells infected with cytomegalovirus (CMV), ganciclovir is first phosphorylated into ganciclovir monophosphate by the protein kinase UL97 of the virus.
It is further phosphorylated by protein kinases in the cell to ganciclovir triphosphate, which is then slowly metabolized in the cell. After removing the extracellular ganciclovir, the half-life of ganciclovir in HSV or HCMV-infected cells was observed to be 18 hours (6-24 hours), respectively. Because the phosphorylation process largely depends on the protein kinase of the virus, the phosphorylation of ganciclovir preferentially occurs in cells infected by the virus. 3. Ganciclovir inhibits virus activity mainly by inhibiting the synthesis of viral DNA: (a) Competitively inhibits viral DNA polymerase, so that deoxyguanosine triphosphate cannot bind to DNA. (B) The binding of ganciclovir triphosphate to the viral DNA terminates or restricts the elongation of the viral DNA chain. The IC50 range of ganciclovir on CMV in vitro is 0.08mcM (0.02ug/mL)-14mcM (3.5ug/mL).
Indications [2]
It is suitable for the treatment of acquired immunodeficiency syndrome (AIDS) patients with cytomegalovirus (CMV) retinitis, as well as the prevention of CMV infection in high-risk solid organ transplant patients.
Usage and dosage [2]
Standard dosage: Valganciclovir hydrochloride tablets should be taken orally with food (see pharmacokinetic characteristics-absorption). Valganciclovir hydrochloride tablets can be quickly and massively converted into ganciclovir. The bioavailability of valganciclovir hydrochloride tablets measured by ganciclovir is 10 times higher than that of ganciclovir capsules. Therefore, the dosage and instructions of valganciclovir hydrochloride tablets described below should be strictly followed.
The absolute bioavailability of valganciclovir hydrochloride tablets measured by ganciclovir is 10 times higher than that of ganciclovir capsules. Valganciclovir hydrochloride tablets cannot replace ganciclovir capsules 1:1. Patients who have previously used ganciclovir capsules to switch to valganciclovir hydrochloride tablets should be informed that if they take more than the prescribed dose of valganciclovir hydrochloride tablets, there is a risk of overdose. (See dosage and usage, overdose). It is recommended to monitor the complete blood count and platelet count during treatment. Patients with severe leukopenia, neutropenia, anemia, and/or thrombocytopenia are recommended to be treated with blood cell growth factor and/or consider suspension of medication. For patients with renal insufficiency, the dose needs to be adjusted according to the creatinine clearance rate.
Reference
[1] CN201610008835.0 A preparation method of ganciclovir
[2] Instructions for Valganciclovir Hydrochloride Tablets
[3] CN201610792340.1 A ganciclovir injection and its preparation process
- Related articles
- Related Qustion
- Ganciclovir: applications and its therapeutic drug monitoring Feb 20, 2025
Ganciclovir is a synthetic nucleoside analog of guanine closely related to acyclovir, but has greater activity against cytomegalovirus.
- Ganciclovir: Antimicrobial Activity and Pharmacokinetics Sep 12, 2024
Ganciclovir is a nucleoside analogue that differs from acyclovir by having an extra hydroxymethyl group on the acyclic side chain.
- What is Ganciclovir? Feb 10, 2020
Ganciclovir is a potent inhibitor of herpes viruses, including CMV. It is a nucleoside analogue that suppresses the replication of herpes viruses and functions as a virustatic agent.
Salmeterol is used as a long-term (maintenance) treatment to prevent or decrease wheezing and trouble breathing caused by asthma or ongoing lung disease (chronic obstructive pulmonary disease-COPD, which includes chronic bronchitis and emph....
Aug 25,2020APILanolin has been used for thousands of years for its ability to soften and relieve dry, painful, and cracked skin. Its popularity peaked in the mid-1900s but is now declining due to its potential to cause skin allergies.....
Aug 27,2020Food AdditivesGanciclovir
82410-32-0You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025
- Ganciclovir
-

- $5.00/ KG
- 2025-12-23
- CAS:82410-32-0
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Ganciclovir
-

- $5.00/ KG
- 2025-12-23
- CAS:82410-32-0
- Min. Order: 0.10000000149011612KG
- Purity: 99% hplc
- Supply Ability: 5000kg
- Ganciclovir
-

- $0.00 / 1Kg/Bag
- 2025-12-23
- CAS:82410-32-0
- Min. Order: 1KG
- Purity: 98%-102% HPLC;USP
- Supply Ability: 500KGS