Luteolin: activities and pharmacokinetics
Dec 18,2023
General Description
Luteolin, a plant-derived flavonoid, exhibits significant anti-inflammatory and anti-cancer effects. It acts on molecular pathways to reduce inflammation by inhibiting key transcription factors and signaling pathways such as NF-κB, AP-1, and STAT3. In cancer, luteolin impedes cell proliferation, induces apoptosis, and reverses epithelial-mesenchymal transition, crucial for metastasis prevention. Pharmacokinetically, luteolin's absorption, distribution, metabolism, and excretion are influenced by its form, dosage, administration route, and individual factors. Enhancements like nanocrystallization improve its bioavailability, with most metabolites excreted renally or biliary within 24 hours. Understanding its pharmacokinetics is vital for optimizing therapeutic use.

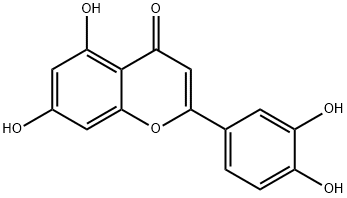
Figure 1. Luteolin
Activities
Anti-inflammatory effects
Luteolinhas garnered attention for its potent anti-inflammatory properties. Ethnopharmacologically, plants rich in luteolin have long been utilized to alleviate inflammation-associated symptoms. Research, including in vitro and in vivo studies, supports the efficacy of both isolated luteolin and extracts from luteolin-rich plants in mitigating inflammatory responses. Mechanistically, luteolin's anti-inflammatory activity is multifaceted, targeting key transcription factors within critical signaling pathways. It modulates the nuclear factor (NF)-κB pathway by inhibiting Src, affects the activator protein (AP)-1 pathway through MAPK, and impacts the signal transducer and activator of transcription 3 (STAT3) pathway by regulating SOCS3. These molecular interactions contribute to the suppression of inflammatory processes. Recent clinical trials have demonstrated promising results, with luteolin-containing formulations showing significant therapeutic effects on inflammation-related diseases. 1
Anti-cancer effects
Luteolin possesses notable anti-cancer properties. It is effective against a spectrum of human cancers including those of the lung, breast, brain (glioblastoma), prostate, colon, and pancreas. Its anti-cancer effects are multifaceted—luteolin hampers tumor cell proliferation, shields cells from carcinogenic factors, induces cell cycle arrest, and triggers apoptosis via different signaling pathways. One key feature of luteolin's action is its ability to reverse the epithelial-mesenchymal transition (EMT), a process crucial for cancer metastasis. It achieves this by causing cytoskeletal contraction, enhancing the expression of the epithelial marker E-cadherin, and reducing the levels of mesenchymal markers such as N-cadherin, snail, and vimentin. Moreover, luteolin increases intracellular reactive oxygen species (ROS) in cancer cells, particularly glioblastoma, leading to lethal endoplasmic reticulum stress and mitochondrial dysfunction. It also stimulates the expression of ER stress-associated proteins including eIF2α, PERK, CHOP, ATF4, and cleaved-caspase 12. These actions collectively contribute to its potential as an anti-cancer agent. 2
Pharmacokinetics
Luteolin, a flavonoid found in many plants, has garnered attention for its potential health benefits. Its pharmacokinetic profile is crucial to understanding its safety and efficacy as a therapeutic agent. Following administration, luteolin absorption is influenced by various factors such as the drug's form, dose, and route of administration, as well as individual gastrointestinal absorption capabilities. Oral administration leads to rapid absorption with peak plasma levels observed within less than an hour, although bioavailability may be low due to factors like poor solubility and extensive first-pass metabolism. The bioavailability of luteolin is notably affected by its limited absorption in the small intestine and significant metabolic activity. It is primarily absorbed through passive diffusion and metabolized by enzymes such as cytochrome P450 present in the intestinal wall. Innovations like nanocrystallization and microemulsion systems have been shown to enhance its water solubility and bioavailability without altering its metabolic profile. Once absorbed, luteolin distributes into various organs, with a higher concentration observed in the liver, spleen, and lungs. Its distribution is partly determined by its affinity for plasma proteins, including human serum albumin, which can restrict its distribution in tissues with lower protein binding capacity. Luteolin also has the capability to cross the blood-brain and placental barriers, allowing it to reach the central nervous system and embryonic tissues. Metabolism of luteolin occurs predominantly in the liver, undergoing processes such as glucuronidation, sulfation, and methylation, producing metabolites like luteolin-3′-O-glucuronide among others. The role of cytochrome P450 enzymes in generating various hydroxylated luteolin derivatives further illustrates the complexity of its metabolic pathways. Excretion of luteolin and its metabolites is mainly via the renal and biliary routes. The cumulative recovery in bile and urine indicates that a significant proportion of luteolin is eliminated through these pathways, with most being excreted within 24 hours post-administration. Glucuronide conjugates are the primary forms detected in urine, accounting for a substantial fraction of total excreted metabolites. Understanding luteolin's pharmacokinetics is essential for dose optimization and therapeutic monitoring in clinical settings, taking into account individual patient factors such as age, gender, and genetic predispositions that may affect drug absorption, distribution, metabolism, and excretion. 3
Reference
1. Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018 Oct 28;225:342-358.
2. Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019 Apr;112:108612.
3. Yao C, Dai S, Wang C, Fu K, Wu R, Zhao X, Yao Y, Li Y. Luteolin as a potential hepatoprotective drug: Molecular mechanisms and treatment strategies. Biomed Pharmacother. 2023 Nov;167:115464.
- Related articles
- Related Qustion
- Luteolin: Overview and its Anticancer Perspectives Apr 30, 2024
Luteolin, a natural compound with antioxidant and anticancer properties, shows promise in inhibiting cancer progression in breast and prostate cancer through various mechanisms
- The effect of dietary Luteolin on glycolipid metabolism disorder Nov 23, 2023
Luteolin, as a natural compound with a wide range of biological activities, are an ideal dietary supplement for maintaining metabolic homeostasis, especially significantly favouring carbohydrate and lipid metabolism.
- Luteolin: mechanism of action and clinical applications Aug 1, 2023
Luteolin inhibits inflammation, tumor growth, and promotes healing. It shows promise in treating skin cancer, wounds, and psoriasis.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCuprous bromide is a white solid compound that turns green in light and a versatile catalyst in polymerization and organic reactions, but its toxicity requires careful handling.....
Dec 18,2023APILuteolin
491-70-3You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025