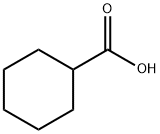
Microbial Biosynthesis and Enzymatic Activation Pathways of Cyclohexanecarboxylic Acid
Nov 24,2025
Shikimate-DerivedFormationof Cyclohexanecarboxylic Acid in Microorganisms
The formation of the cyclohexanecarboxylic acid starter unit of ω-cyclohexyl fatty caids from shikimic acid in Alicyclobacillus acidocaldarius (formerly Bacillus acidocaldarius) has been studied. Cyclohexanecarboxylic acid derivatives are also encountered in two examples of secondary metabolites in Streptomyces. Firstly, cyclohexanecarboxylic acid is found attached via a D-alanine unit to the macrocycle of the ansamycin antibiotic ansatrienin A from Streptomyces collinus11 and to the related trienomycins. Secondly, a singly substituted cyclohexane ring is found in asukamycin, produced by S. nodosus, at the terminus of a short polyene chain. [1]
Stepwise Non-Aromatic Conversion Pathway from Shikimic Acid to CCA
The metabolic steps for the conversion of shikimic acid to cyclohexanecarboxylic acidin S. collinus have been established. The pathway involves a series of dehydrations and double bond reductions interspersed in such a way that no intermediate is ever aromatic. 1, 4-Conjugate elimination of the hydroxy group at C-3 and a proton from C-6 of shikimic acid gives rise to a cross-conjugated diene, which undergoes reduction of the double bondconjugated to the carbonyl group. Another 1,4-elimination involving the C-4 hydroxy group and the proton at C-l gives a5-hydroxy 1,3-diene. Reduction to 5-hydroxycyclohex-1 -ene-carboxylic acid proceeds either directly or via the 2 isomer. Another reduction gives the hydroxy acid, which undergoesdehydration involving a nonactivated proton. Isomerization of the double bond into conjugation and a final reduction completesthe sequence. At least some of the reactions take place at thelevel of the corresponding thioesters. [1]
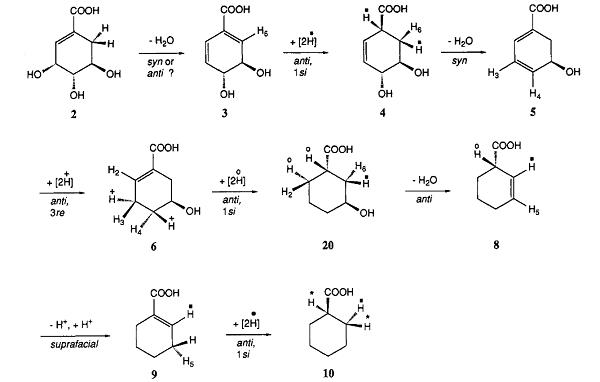
Microbial β-Oxidation–Based Degradation Pathway of Cyclohexanecarboxylic Acid
The metabolism of cyclohexanecarboxylic acid by a bacterium, designated PRL W19, follows a pathway involving β-oxidation of coenzyme A intermediates analogous to the classical oxidation of fatty acids. The organism appears to have the property for the constitutive metabolism of caproic acid, and cell extracts contain high levels of the enzymes required for the functioning of the fatty acid cycle. However, the metabolism of cyclohexanecarboxylic acid requires induction by growth or incubation with an appropriate substrate. Extracts of induced cells contain several enzyme activities which are synthesized in response to the induction process. These enzymes include cyclohexanecarboxyl-CoA synthetase, cyclohexanecarboxyl-CoA dehydrogenase, 1-cyclohexenecarboxyl-CoA hydratase, and trans-2-hydroxycyclohexanecarboxyl-CoA dehydrogenase. A characteristic feature of this organism is that it becomes induced for the metabolism of benzoate and catechol during growth on cyclohexanecarboxylic acid, but benzoate does not appear to be an obligatory intermediate in the metabolism of cyclohexanecarboxylic acid. [2]
Cyclohexanecarboxylate as the Starter Unit in Phoslactomycin Biosynthesis
Phoslactomycins (PLMs) A–F, produced by actinomycetes are polyketide-type antibiotics derived from a hydroxycyclohexanecarboxylic acid or a cyclohexanecarboxylic acid starter unit. Feeding experiments with [2-13C]shikimic acid indicated that the C-18 carbon of PLMs comes from C-5 of shikimate. Further feeding studies of cis and trans-3-hydroxy[7-13C]cyclohexanecarboxylic acid, [7-13C]- and [2H11]cyclohexanecarboxylic acid have suggested that the starter unit in the PLM biosynthesis is not cis-3-hydroxycyclohexanecarboxylate but cyclohexanecarboxylate and that PLM-B is produced initially, and subsequently converted to other analogs by hydroxylation and acylation. [3]
CHC-CoA Biosynthesis via Promiscuous Shikimoyl-CoA Synthetase and FAD-Dependent Dehydratase
Study elucidates a microbial biosynthetic route leading to cyclohexanecarboxyl-CoA (CHC-CoA), revealing two previously unrecognized enzymatic functions within the shikimate-derived metabolic network. The work demonstratesthata shikimoyl-CoA synthetase exhibits broad substrate promiscuity, activating not only aromatic acids but also cycloalkyl substrates such as cyclohexanecarboxylic acid. In parallel, an enzyme initially annotated as an acyl-CoA dehydrogenase is shown instead to function as an FAD-dependent dehydratase, catalyzing a key dehydration step in CHC-CoAformation. Together, these findings outline a viable biochemical pathwaythatconverts shikimate-derived intermediates into CHC-CoA and expand current understanding of how microorganisms process cyclohexyl-based acids. The pathway offers new enzymatic entry points for metabolic engineering, biotransformationof cycloalkyl carboxylic acids, and the design of biosynthetic routes incorporating CHC-derived building blocks. [4]
References:
[1] Moore, B. S., Poralla, K., & Floss, H. G. (1993). Biosynthesis of the cyclohexanecarboxylic acid starter unit of. omega.-cyclohexyl fatty acids in Alicyclobacillus acidocaldarius. Journal of the American Chemical Society, 115(12), 5267-5274.
[2] E. R. Blakley. 1978. The microbial degradation of cyclohexanecarboxylic acid by a β-oxidation pathway with simultaneous induction to the utilization of benzoate. Canadian Journal of Microbiology. 24(7): 847-855. https://doi.org/10.1139/m78-141
[3] Sekiyama, Y., Palaniappan, N., Reynolds, K. A., & Osada, H. (2003). Biosynthesis of phoslactomycins: cyclohexanecarboxylic acid as the starter unit. Tetrahedron, 59(38), 7465-7471.
[4] Skyrud, W., Flores, A. D. R., & Zhang, W. (2020). Biosynthesis of cyclohexanecarboxyl-CoA highlights a promiscuous shikimoyl-CoA synthetase and a FAD-dependent dehydratase. ACS Catalysis, 10(5), 3360-3364.
- Related articles
- Related Qustion
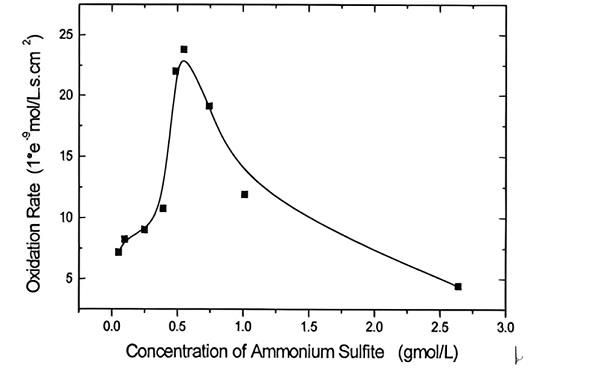
Investigation about ammoniacal leaching of LIB metals and the kinetic behavior of ammonium sulfite oxidation.....
Nov 24,2025Inorganic chemistry2-Adamantanol and its derivatives have specific biological activity and are used for the production of drugs with a wide range of antiviral activity.....
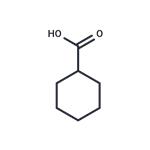
Nov 24,2025APICyclohexanecarboxylic acid
98-89-5You may like
Cyclohexanecarboxylic acid manufacturers
- Cyclohexanecarboxylic acid
-
- 2025-12-14
- CAS:98-89-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Cyclohexanecarboxylic Acid
-
- $44.00 / 5g
- 2025-12-13
- CAS:98-89-5
- Min. Order:
- Purity: 98.13%
- Supply Ability: 10g
- Cyclohexanecarboxylic acid
-

- $0.00 / 25Kg/Drum
- 2025-12-12
- CAS:98-89-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year