Phenylboronic acid:Synthesis,reactions
May 9,2023
Phenylboronic acid is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis including numerous cross coupling reactions.

Synthesis
Numerous methods exist to synthesize phenylboronic acid. One of the most common synthesis uses phenylmagnesium bromide and trimethyl borate to form the ester PhB(OMe)2, which is then hydrolyzed to the product.
PhMgBr + B(OMe)3 → PhB(OMe)2 + MeOMgBr
PhB(OMe)2 + H2O → PhB(OH)2 + MeOH
Other routes to phenylboronic acid involve electrophilic borates to trap phenylmetal intermediates from phenyl halides or from directed ortho-metalation.Phenylsilanes and phenylstannanes transmetalate with BBr3, followed by hydrolysis form phenylboronic acid. Aryl halides or triflates can be coupled with diboronyl reagents using transition metal catalysts. Aromatic C-H functionalization can also be done using transition metal catalysts.
Reactions
The dehydration of boronic acids gives boroxines, the trimeric anhydrides of phenylboronic acid. The dehydration reaction is driven thermally, sometimes with a dehydration agent.
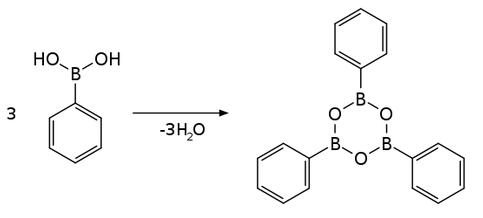
Phenylboronic acid participates in numerous cross coupling reactions where it serves as a source of a phenyl group. One example is the Suzuki reaction where, in the presence of a Pd(0) catalyst and base, phenylboronic acid and vinyl halides are coupled to produce phenyl alkenes.This method was generalized to a route producing biaryls by coupling phenylboronic acid with aryl halides.
C-C bond forming processes commonly use phenylboronic acid as a reagent. Alpha-amino acids can be generated using the uncatalyzed reaction between alpha-ketoacids, amines, and phenylboronic acid.Heck-type cross coupling of phenylboronic acid and alkenes and alkynes has been demonstrated.
Aryl azides and nitroaromatics can also be generated from phenylboronic acid.Phenylboronic acid can also be regioselectively halodeboronated using aqueous bromine, chlorine, or iodine:
Boronic esters result from the condensation of boronic acids with alcohols. This transformation is simply the replacement of the hydroxyl group by alkoxy or aryloxy groups.This reversible reaction is commonly driven to product by the use of Dean-Stark apparatus or a dehydration agent to remove water.
PhB(OH)2 + 2 ROH ⇌ PhB(OR)2 + 2H2O
As an extension of this reactivity, PhB(OH)2 can be used as a protecting group for diols and diamines. This reactivity is the basis of the use of phenylboronic acid's use as a receptor and sensor for carbohydrates, antimicrobial agents, and enzyme inhibitors, neutron capture therapy for cancer, transmembrane transport, and bioconjugation and labeling of proteins and cell surface.
- Related articles
- Related Qustion
- The widely applications of Phenylboronic acid. Apr 30, 2024
Phenylboronic acid, a non-toxic compound, is a catalyst for an efficient, rapid, and one-pot three-component synthesis of tetrahydrobenzo[b]pyrans in good to excellent yields.
- Correlational research of Phenylboronic acid Nov 12, 2019
Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron.
Phenylboronic acid
98-80-6You may like
Phenylboronic acid manufacturers
- Phenylboronic acid
-
- 2025-12-21
- CAS:98-80-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Phenylboronic acid
-
- $130.00 / 100g
- 2025-12-19
- CAS:98-80-6
- Min. Order: 100g
- Purity: 0.99
- Supply Ability: 100kg
- Phenylboronic acid
-

- $0.00 / 200Kg/Drum
- 2025-12-18
- CAS:98-80-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year






