Cupferron: properties, use and application research
Dec 19,2025
Properties and Use
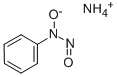
Cupferron (Figure 1) is the ammonium salt of N-nitroso-N-phenylhydroxylamine and exists as a creamy-white crystalline solid at room temperature. It is soluble in water, alcohol, and ether. Cupferron can produce irritating, corrosive, or toxic gases as combustion products. Physical and chemical properties of cupferron are listed in the following table 1. Cupferron is an analytical reagent that complexes with metal ions andis used to separate and precipitate metals such as copper, iron, vanadium, and thorium. It is used to separate tin from zinc and to separate copper and iron from other metals. In analytical laboratories, cupferron is a reagent used for quantitative determination of vanadates and titanium and the colorimetric determination of aluminum. [1]


Carcinogenicity
Cupferron is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Cupferron impairs the growth and virulence of Escherichia coli clinical isolates
Multidrug resistance is a worrying problem worldwide. The lack of readily available drugs to counter nosocomial infections requires the need for new interventional strategies. Drug repurposing represents a valid alternative to using commercial molecules as antimicrobial agents in a short time and with low costs. Contextually, the present study focused on the antibacterial potential of the ammonium salt N-nitroso-N-phenylhydroxylamine (Cupferron), evaluating the ability to inhibit microbial growth and influence the main virulence factors. Cupferron cytotoxicity was checked via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and hemolysis assays. The antimicrobial activity was assessed through the Kirby-Bauer disk diffusion test, broth microdilution method, and time-killing kinetics. Furthermore, the impact on different stages of the biofilm life cycle, catalase, swimming, and swarming motility was estimated via MTT and crystal violet (CV) assay, H2O2 sensitivity, and motility tests, respectively. Cupferron exhibited <15% cytotoxicity at 200 µg/mL concentration. The 90% bacterial growth inhibitory concentrations (MIC90) values recorded after 24 hours of exposure were 200 and 100 µg/mL for multidrug-resistant (MDR) and sensitive strains, respectively, exerting a bacteriostatic action. Cupferron-treated bacteria showed increased susceptibility to biofilm production, oxidative stress, and impaired bacterial motility in a dose-dependent manner. In the new antimicrobial compounds active research scenario, the results indicated that Cupferron could be an interesting candidate for tackling Escherichia coli infections.[2]
Antifungal spectrum of Cupferron against Candida albicans
Candida albicans is an opportunistic yeast accounting for about 50-90 % of all cases of candidiasis in humans, ranging from superficial to systemic potentially life-threatening infections. The presence of several virulence factors, including biofilm, hyphal transition, and proteolytic enzymes production, worsens the fungal infections burden on healthcare system resources. Hence, developing new bioactive compounds with antifungal activity is a pressing urgence for the scientific community. In this perspective, we evaluated the anti-Candida potential of the N-Nitroso-N-phenylhydroxylamine ammonium salt (cupferron) against standard and clinical C. albicans strains. Firstly, the in vitro cytotoxicity of cupferron was checked in the range 400-12.5 μg/mL against human microglial cells (HMC-3). Secondly, its antifungal spectrum was explored via disk diffusion test, broth-microdilution method, and time-killing curve analysis, validating the obtained results through scanning electron microscopy (SEM) observations. Additionally, we evaluated the cupferron impact on the main virulence determinants of Candida albicans. At non-toxic concentrations (100-12.5 μg/mL), the compound exerted interesting anti-Candida activity, registering a minimum inhibitory concentration (MIC) between 50 and 100 μg/mL against the tested strains, with a fungistatic effect until 100 μg/mL. Furthermore, cupferron was able to counteract fungal virulence at MIC and sub-MIC values (50-12.5 μg/mL). These findings may propose cupferron as a new potential antifungal option for the treatment of Candida albicans infections.[3]
The reaction of nitroxyl (HNO) with nitrosobenzene gives cupferron
Nitroxyl (HNO), a penultimate product in the NOS-catalyzed conversion of L-arginine to L-citrulline, generated from Angeli's salt (AS) was determined by trapping it with nitrosobenzene (NB) to produce cupferron. The cupferron thus produced was characterized by complexation with Fe3+, Al3+, Cu2+, or Sn2+. UV/VIS spectra of the solubilized (in CHCl3) precipitates formed from NB and nitroxyl generated from AS in the presence of the iron, aluminum, copper, or tin salts were identical to those of their corresponding cupferron complexes. The identities of the Fe3+ and Cu2+ complexes formed from NB and HNO were further confirmed by their identical retention times on HPLC when compared to authentic Fe3+ and Cu2+ cupferron complexes. It was possible to detect 5 x 10-6 M of the cupferron Fe3+ complex spectrophotometrically and to measure its production from the nitroxyl generators AS and methanesulfohydroxamic acid (MSHA) in the presence of 10(-4) M NB. The yield of cupferron was 51 and 62% of the amount of nitroxyl possible from AS or MSHA, respectively, after taking into account the relative rates of nitroxyl generation from these donors.[4]
References
[1] National Toxicology Program. Cupferron. Rep Carcinog. 2011;12:123-124.
[2] Palma F, Dell'Annunziata F, Folliero V, et al. Cupferron impairs the growth and virulence of Escherichia coli clinical isolates. J Appl Microbiol. 2023;134(10):lxad222. doi:10.1093/jambio/lxad222
[3] Palma F, Acunzo M, Della Marca R, et al. Evaluation of antifungal spectrum of Cupferron against Candida albicans. Microb Pathog. 2024;194:106835. doi:10.1016/j.micpath.2024.106835
[4] Shoeman DW, Nagasawa HT. The reaction of nitroxyl (HNO) with nitrosobenzene gives cupferron (N-nitrosophenylhydroxylamine). Nitric Oxide. 1998;2(1):66-72. doi:10.1006/niox.1998.0166
- Related articles
- Related Qustion
Sertraline is a naphthalenamine derivative with the predominant pharmacological action of inhibiting presynaptic reuptake of serotonin from the synaptic cleft.....
Dec 19,2025DrugsTrimethyloxonium Tetrafluoroborate serves as a strong electrophilic methylating agent, mainly used for the methylation of hydroxyl groups.....
Dec 19,2025API