Property, Synthesis and Clinical Effect of 5-aminolevulinic acid hydrochloride
Jul 12,2022
General description
The CAS number of 5-aminolevulinic acid hydrochloride is 5451-09-2, the molecular formula is C5H10ClNO3, the molecular weight is 167.5908 [1], the molar refractive index is 30.34, the molar volume (m3/mol) is 106.4, the constant tension specific volume (90.2K) is 286.9 [2], the surface tension (DYNE /cm) is 52.7, and the polarization (10-24cm3) is 12.02 [3].
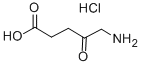
Fig. 1 The structure of 5-aminolevulinic acid hydrochloride.
Synthesis
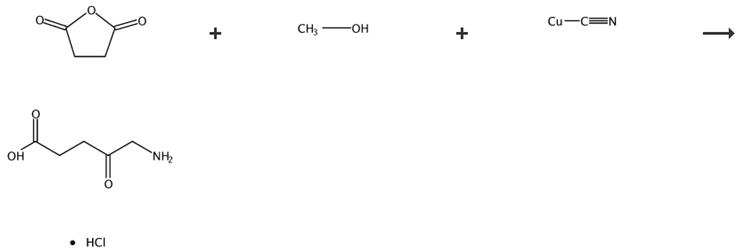
Fig. 2 The synthetic route of 5-aminolevulinic acid hydrochloride [4].
5-Aminolevulinic acid hydrochloride (3). A stirred mixture of compound 2 (5.4 g) was hydrogenated by the addition of Pd/C (0.5 g) in 6 M HCI under a pressure of 125 psi at room temperature for 18 h. The resulting mixture was filtered through celite, and the organic solution concentrated at reduced pressure, yielding the crude product. The crude product was purified, by recrystallization with EtOH/2-propanol. Product 3 as a solid, yield 5.7 g, 89%.1H-NMR (200 MHz, D2O), δ(ppm): 4.13 (d, 2H), 2.90 (t, 2H), 2.72 (t, 2H), 13C-NMR (50 MHz, DMSO-d6) δ 172.3; 48.2; 35.2; 31.0, ESI MS: m/z168.04 [M]+.
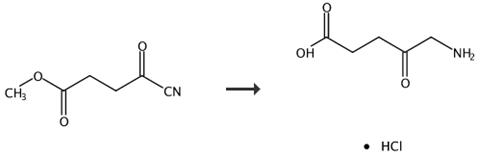
Fig. 3 The synthetic route of 5-aminolevulinic acid hydrochloride [5].
5-Aminolevulinic acid hydrochloride (3). A stirred mixture of compound 2 (5.4 g) was hydrogenated by the addition of Pd/C (0.5 g) in 6 M HCI under a pressure of 125 psi at room temperature for 18 h. The resulting mixture was filtered through celite, and the organic solution concentrated at reduced pressure, yielding the crude product. The crude product was purified, by recrystallization with EtOH/2-propanol. Product 3 as a solid, yield 5.7 g, 89%. 1H-NMR (200 MHz, D2O), δ(ppm): 4.13 (d, 2H), 2.90 (t, 2H), 2.72 (t, 2H), 13C-NMR (50 MHz, DMSO-d6) δ 172.3; 48.2; 35.2; 31.0, ESI MS: m/z168.04 [M]+.
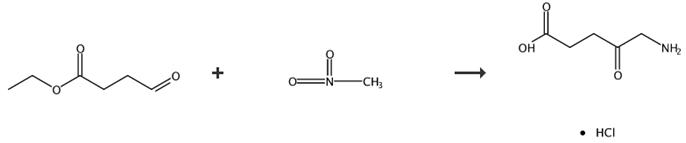
Fig. 4 The synthetic route of 5-aminolevulinic acid hydrochloride [6].
Synthesis of 5-aminolevulinic acid hydrochlorate (4) 3 (0.19 g, 1 mmol), 10 mL hydrochloric acid (1 mol·L-1) and 10 % Pd/C (0.11 g, 0.1 mmol) were added into a 30 mL reaction flask followed by nitrogen gas under ordinary pressure. The reaction was performed for 24 h and then ceased. The resulting solution was filtered and desolventized. The residue was recrystallized from ethanol/acetone to give the desired product as a brownish yellow solid (0.15 g, yield 91 %). 5-aminolevulinic acid hydrochlorate (4), 91 %, brownish solid m.p. 143-144 °C; 1H NMR (D2O, 400 MHz) δ: 2.61 (t, J = 6.2 Hz, 2H, CH2CO2H), 2.79 (t, J = 6.0 Hz, 2H, CH2CO), 4.02 (s, 2H, CH2NH3+); IR (KBr) ν: 3126, 1725, 1403, 1178, 1049 cm-1; MS m/z: 132 (100, M+-HCl). Anal. calcd for C5H10NClO3: C 35.83, H 6.01, N 8.36; found C 35.59, H 6.12, N 8.36.
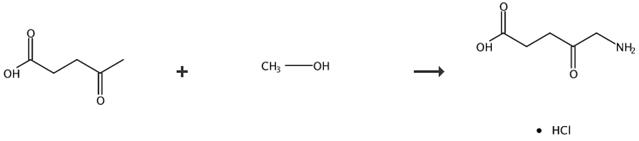
Fig. 5 The synthetic route of 5-aminolevulinic acid hydrochloride [7].
Conversion of 5-aminolevulinic acid hydrochloride by catalytic reduction of the 5-azidolevulinic acid alkyl esters and subsequent hydrolysis of the intermediate 5-aminolevulinic acid hydrochlorides. General procedure for the preparation and the hydrolysis of the intermediate 5-aminolevulinic acid ester hydrochlorides, which contain ester alkyl chains C1-C3 by catalytic hydrogenation of the alkyl esters and subsequent acid catalysed hydrolysis with formation of 5-aminolevulinic acid hydrochloride. Azidolevulinic acid ester (1 g) was dissolved in a mixture of the alcohol (10 ml), which represents the alkyl chain in the final ester) hydrochloric acid in water (2 mol/l), and the mixture was treated with a hydrogenation catalyst (palladium on charcoal). The mixture was hydrogenated while passing in hydrogen at a pressure of 1-6 bar for 3 h. By monitoring the hydrogenation, it was found that the hydrogenation was completed quantitatively after 3 h (1H-NMR). The hydrogenation is accompanied by an increase of the reaction temperature to 35°C. The reaction is complete, when the reaction temperature reaches 20-25°C again (1H-NMR). The catalyst was then filtered from the reaction mixture, and the alcohol was distilled in vacuo. A small amount of activated charcoal and aqueous hydrochloric acid (10 ml, 6 mol/l) were added and subsequently the reaction mixture was refluxed for 5 h. The activated charcoal was then filtered, and both the water and the alcohol were removed by distillation in vacuo. While stirring, to the nearly colorless and viscous residue 2-propanol (20 ml) was added. After one minute a white and crystalline solid abruptly precipitated. The solid was filtered using a glass frit Washing of the solid with small amount of 2-propanol and drying of the crystals in vacuo. yielded colorless crystals (85-90%) consisting of pure 5-aminolevulinic acid hydrochloride. Melting point 150-151°C.
Study in vitro
5-Aminolevulinic acid hydrochloride (ALA) is a non-fluorescent prodrug that leads to intracellular accumulation of fluorescent porphyrins in malignant gliomas-a finding that is under investigation for intraoperative identification and resection of these tumours. Median follow-up was 35.4 months (95% CI 1.0-56.7). Contrast-enhancing tumour was resected completely in 90 (65%) of 139 patients assigned 5-aminolevulinic acid compared with 47 (36%) of 131 assigned white light (difference between groups 29% [95% CI 17-40], p<0.0001) [8]. Patients allocated 5-aminolevulinic acid had higher 6-month progression free survival than did those allocated white light (41.0% [32.8-49.2] vs 21.1% [14.0-28.2]; difference between groups 19.9% [9.1-30.7], p=0.0003, Z test) [9]. 5-ALA alone proved to be insufficient in attaining gross total resection without the danger of incurring postoperative neurological deterioration. Furthermore, in the case of functional grade III gliomas, iMRI in combination with functional neuronavigation was significantly superior to the 5-ALA resection technique.
Appliacation
The physiological substance and precursor of the heme synthesis 5-aminolevulinic acid (ALA) is a promising prodrug for photodiagnosis and photodynamic therapy of epithelial tumors, particularly in urological and gynecological tissues. Experiments show that the low pH ALA aqueous solution may be one possible dosage form to be considered for market introduction [10].
Surgical resection is typically the first line of treatment for gliomas. However, the neurosurgeon faces a major challenge in achieving maximal resection in high-grade gliomas as these infiltrative tumors make it difficult to discern tumor margins from normal brain with conventional white-light microscopy alone. To aid in resection of these infiltrative tumors, fluorescence-guided surgery has gained much popularity in intraoperative visualization of malignant gliomas, with 5-aminolevulinic acid (5-ALA) leading the way [11]. First introduced in an article in Neurosurgery, 5-ALA has since become a safe, effective, and inexpensive method to visualize and improve resection of gliomas. This has undoubtedly led to improvements in the clinical course of patients as demonstrated by the increased overall and progression-free survival in patients with such devastating disease. This literature review aims to discuss the major studies and trials demonstrating the clinical utility of 5-ALA and its ability to aid in complete resection of malignant gliomas [12].
Clinical effect
5-ALA is a part of the heme biosynthetic pathway found in all living mammalian cells. As part of this pathway, 5-ALA is metabolized and transformed through a series of reactions in the mitochondria into protoporphyrin IX (PpIX), a fluorescent metabolite. In physiologically normal cells, PpIX is chelated by ferrochelatase into heme [13].
Exogenous 5-ALA acts as an orally administered “prodrug” and has demonstrated marked penetration of the blood–brain barrier (BBB), most notably in areas with a high density of malignant cells [14]. There are many theories describing the mechanisms of and reasoning for accumulation of 5-ALA and PpIX in malignant cells. Decreased ferrochelatase levels, increased uptake of PpIX by ATP-binding cassette B6, increased cellular density, increased proliferative activity by malignant tumor cells, increased angiogenesis, and increased BBB permeability in areas of malignancy have all been implicated in increased fluorescence of gliomas [15]. In normal cells, the synthesis of PpIX is regulated by a feedback control system (therefore avoiding its accumulation), and it has been suggested that this feedback system can be set out of control when high amounts of exogenous 5-ALA are administered [16].
Storage methods
Keep sealed in a cool and dry place under 2-8℃, away from light.
Ecological data
Generally, it is not harmful to water. Do not discharge materials into the surrounding environment without government permission.
Properties and stability
Stable at room temperature and pressure.
References
[1] A. Bunke, O. Zerbe, H. Schmid, G. Burmeister, H. Merkle, B. Gander, Degradation mechanism and stability of 5‐aminolevulinic acid, Journal of pharmaceutical sciences 89(10) (2000) 1335-1341.
[2] L. Cottier, G. Descotes, L. Eymard, K. Rapp, Syntheses of γ-oxo acids or γ-oxo esters by photooxygenation of furanic compounds and reduction under ultrasound: application to the synthesis of 5-aminolevulinic acid hydrochloride, Synthesis 1995(03) (1995) 303-306.
[3] B. Elfsson, I. Wallin, S. Eksborg, K. Rudaeus, A. Ros, H. Ehrsson, Stability of 5-aminolevulinic acid in aqueous solution, European journal of pharmaceutical sciences 7(2) (1999) 87-91.
[4] M.K. Fehr, C.F. Chapman, T. Krasieva, B.J. Tromberg, J.L. McCullough, M.W. Berns, Y. Tadir, Selective photosensitizer distribution in vulvar condyloma acuminatum after topical application of 5-aminolevulinic acid, American journal of obstetrics and gynecology 174(3) (1996) 951-957.
[5] M. Fujino, Y. Nishio, H. Ito, T. Tanaka, X.-K. Li, 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction, International Immunopharmacology 37 (2016) 71-78.
[6] M.H. Gold, The evolving role of aminolevulinic acid hydrochloride with photodynamic therapy in photoaging, Cutis 69(6 Suppl) (2002) 8-13.
[7] H.-J. Ha, S.-K. Lee, Y.-J. Ha, J.-W. Park, Selective bromination of ketones. A convenient synthesis of 5-aminolevulinic acid, Synthetic communications 24(18) (1994) 2557-2562.
[8] S.H. Halani, D.C. Adamson, Clinical utility of 5-aminolevulinic acid HCl to better visualize and more completely remove gliomas, OncoTargets and therapy 9 (2016) 5629.
[9] S. Ito, N. Miyoshi, W.G. Degraff, K. Nagashima, L.J. Kirschenbaum, P. Riesz, Enhancement of 5-aminolevulinic acid-induced oxidative stress on two cancer cell lines by gold nanoparticles, Free radical research 43(12) (2009) 1214-1224.
[10] H. Kawakami, T. Ebata, H. Matsushita, A new synthesis of 5-aminolevulinic acid, Agricultural and biological chemistry 55(6) (1991) 1687-1688.
[11] D. Ormrod, B. Jarvis, Topical aminolevulinic acid HCl photodynamic therapy, American journal of clinical dermatology 1(2) (2000) 133-139.
[12] M.E. Rocha, F. Dutra, B. Bandy, R.L. Baldini, S.L. Gomes, A. Faljoni-Alário, C.W. Liria, M. Terêsa, M. Miranda, E.J. Bechara, Oxidative damage to ferritin by 5-aminolevulinic acid, Archives of Biochemistry and Biophysics 409(2) (2003) 349-356.
[13] W.G. Solis, M. Hansen, Fluorescence in a cryptococcoma following administration of 5-aminolevulinic acid hydrochloride (Gliolan), Case Reports 2017 (2017) bcr-2017-219469.
[14] K. Watanabe, T. Tanaka, Y. Hotta, H. Kuramochi, Y. Takeuchi, Improving salt tolerance of cotton seedlings with 5-aminolevulinic acid, Plant Growth Regulation 32(1) (2000) 97-101.
[15] S.R. Wiegell, H.C. Wulf, Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate, Journal of the American Academy of Dermatology 54(4) (2006) 647-651.
[16] V. Yadav, Y. Mai, L.E. McCoubrey, Y. Wada, M. Tomioka, S. Kawata, S. Charde, A.W. Basit, 5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease, Biomedicines 9(5) (2021) 578.
- Related articles
- Related Qustion
- 5-Aminolevulinic acid hydrochloride: From Therapeutic Potential in Oncology to Nephroprotection Mar 28, 2025
5-Aminolevulinic acid hydrochloride finds an important role as a precursor in the synthesis of tetrapyrroles such as chlorophyll and heme.
- The uses of 5-Aminolevulinic acid hydrochloride Jul 15, 2024
5-Aminolevulinic acid (5-amino-4-oxopentanoic acid, ALA) hydrochloride is widely used for fluorescence diagnosis and photodynamic therapy of malignant tumours and treating skin and other diseases.
3-Hydroxytyramine hydrochloride is white or white shiny crystal; Odorless, slightly bitter taste; When exposed to air and light, the color gradually deepens.....
Jul 12,2022APIBenzophenone is a common fine chemical intermediate and additive, which is widely used in pesticides, pharmaceuticals, electronic chemicals and many other fields.....
Jul 12,2022API5-Aminolevulinic acid hydrochloride
5451-09-2You may like
5-Aminolevulinic acid hydrochloride manufacturers
- 5-Aminolevulinic acid hydrochloride
-
- $46.00 / 100mg
- 2025-12-18
- CAS:5451-09-2
- Min. Order:
- Purity: 99.97%
- Supply Ability: 10g
- 5-Aminolevulinic acid hydrochloride
-

- 2025-12-18
- CAS:5451-09-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 5-Aminolevulinic acid hydrochloride
-

- $0.00 / 5g
- 2025-12-18
- CAS:5451-09-2
- Min. Order: 5g
- Purity: ≥98.0%
- Supply Ability: 10kg/month