Side-effects of Nalidixic acid
Mar 11,2022
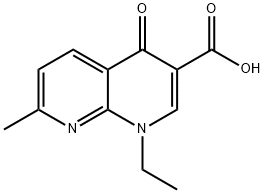
Nalidixic acid (1-ethyl-l,4-dihydro-7-methyl-4-oxo-1, 8-naphthyridine- 3-carboxylic acid) is one of a series of 1,8-naphthyridine derivatives which were first synthesized by Lesher et al.from a distillate during chloroquine synthesis. Nalidixic acid was introduced into clinical use in 1964 and has a significant historical role as the prototype of the quinolone group of drugs.
Mechanism of action
Nalidixic acid inhibits bacterial DNA replication by inhibition of type II topoisomerases, DNA gyrase, and topoisomerase IV, causing DNA degradation and inducing filamentation. DNA topoisomerases control the configuration of chromosomal DNA to facilitate replication, recombination, and expression by improved efficiency in the breaking and rejoining of DNA strands. Type I topoisomerases cleave one DNA strand, while type II topoisomerases cleave both strands. These enzymes are required to supercoil strands of bacterial DNA so that they fit in the bacterial cell.
In order to introduce negative (under wound) superhelical turns, DNA gyrase must impose a twisting stress and carry out a nicking–closing reaction to relieve the positive winding stress. Quinolones interrupt the function of DNA gyrase in which it breaks and rejoins DNA strands, such that DNA strand-breaking occurs, but the rejoining of broken strands is blocked. In general, the primary target for quinolones in Gram-negative bacteria is DNA gyrase and in Grampositive bacteria is topoisomerase IV. DNA gyrase contains two A and two B subunits. Gyrase A protein mediates the breakage and rejoining of DNA, while gyrase B protein mediates the ATPase activity of the enzyme and therefore functions principally in energy transduction and ATP hydrolysis during gyrase function.
Clinical uses
Nalidixic acid has now been largely replaced in clinical usage by the newer fluoroquinolones that have superior in vitro activity and improved pharmacokinetic and pharmacodynamic profiles. Nevertheless, nalidixic acid remains useful for some indications.
Nalidixic acid is useful for treatment of urinary tract infections caused by organisms such as E. coli and all species of Enterobacter, Klebsiella, and Proteus, although its in vitro activity against common pathogens encountered in patients with complicated and/or nosocomial urinary tract infections is significantly less than that of most fluoroquinolones.
Nalidixic acid has been used for selective decontamination of the digestive tract (SDD) to prevent infection in neutropenic patients.
Side-effects
Gastrointestinal side-effects Nausea, vomiting, or diarrhea are infrequent, mild, and reversible.
Neurotoxicity
This is uncommon and includes visual disturbances, excitement, depression, confusion, and hallucinations. Amfenolic acid, a derivative of nalidixic acid, was originally developed as a central nervous system stimulant. Headache, giddiness, insomnia, drowsiness, syncope, and sensory changes have also been described. Visual disturbances include excessive brightness of lights, blurred vision, difficulty in focusing, decreased visual acuity, diplopia, and alteration in color perception.
Convulsions have occurred in small numbers of patients. Acute reversible psychosis has been observed in a patient treated with large doses of nalidixic acid. Similarly, Kremer et al. described an adult patient with acute glomerulonephritis who developed a paranoid state after treatment with nalidixic acid for a urinary infection.
Severe neurotoxic reactions due to nalidixic acid have usually
occurred when this drug has been used in large doses. It is wise to use
it cautiously in patients with pre-existing mental instability, epilepsy,
and cerebral arteriosclerosis. Among the newer fluoroquinolones,
neurological symptoms have been reported in 0.9–7.4% of patients in
clinical trials.
- Related articles
- Related Qustion
- What is nalidixic acid used for? Sep 10, 2024
Nalidixic acid has a bactericidal or bacteriostaic effect depending on the sensitivity of the microorganism and the concentration.
Tinidazole, 1-[2-(ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole, is a nitroimidazole drug similar to metronidazole. It was synthesized in 1969 and it has a similar activity profile as metronidazole in vitro, including efficacy against Tric....
Mar 11,2022APICiprofloxacin is a fluoroquinolone (also called 4-quinolone, or quinolone carboxylic acid) which was developed by Bayer Pharmaceuticals for both oral and parenteral use. It is one of the second generation of quinolones (others include norfl....
Mar 11,2022APINalidixic acid
389-08-2You may like
- Nalidixic acid
-
- $0.00 / 1kg
- 2025-12-20
- CAS:389-08-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Nalidixic acid
-

- $0.00 / 1removed
- 2025-12-15
- CAS:389-08-2
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g
- Nalidixic acid
-

- $0.00 / 1removed
- 2025-12-15
- CAS:389-08-2
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g