Stability of Nicotinamide riboside chloride and chemical properties of its derivatives
Nov 16,2023
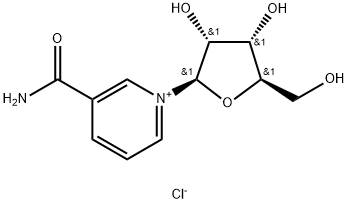
Introduction of Nicotinamide riboside chloride
Nicotinamide Riboside is an orally active NAD precursor that increases NAD levels and activates SIRT1 and SIRT3. It occurs naturally in milk and is already available as a nutritional supplement. Oral NR has been shown to increase NAD levels in a variety of tissues, while increasing SIRT activity, improving mitochondrial function and the regenerative potential of stem cells. Nicotinamide riboside chloride (NRCl) is a potent form of vitamin b3. However, it cannot be used in ready-to-drink (RTD) beverages or high-water active foods due to its instability in water. In addition, NR is currently considered a favorable precursor because NR has no serious side effects or flushes compared to other NAD precursors.

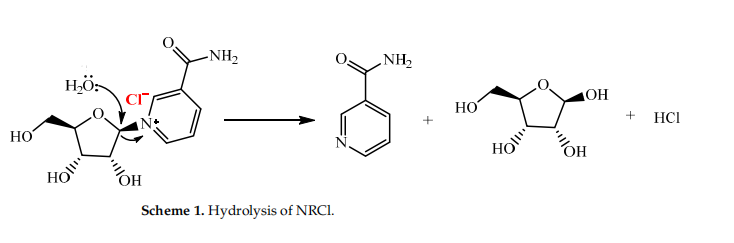
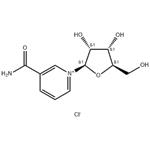
Hydrolysis of NRCl
NRCl is easily hydrolyzed in an aqueous solution and is, therefore, unsuitable for incorporation into beverages, and other foods and supplements, with highwater activity. Structurally, NRCl is a quaternary ammonium salt containing a sensitive N-glycosidic bond that can spontaneously cleave in an aqueous solution, yielding nicotinamide and D-ribose decomposition products (Scheme 1). This means that it will be difficult to develop NRCl for ready-to-drink (RTD) beverages or high-water activity foods or supplements.
Synthesis of NRTOCl as a new oil-soluble hydrophobic NR derivative
In order to make NRCl more stable in aqueous solution without losing its role as a NAD+ precursor. Therefore, the oil-soluble hydrophobic compound nucleoside trioleate chloronicotinamide (NRTOCl) was synthesised. The synthetic design of NRTOCl was predicated on the idea that the long arms of the oleate esters would provide protection to the N-glycosidic bond from hydrolysis in aqueous solutions.
A new compound of NRTOCl was synthesized by reacting NRCl with oleyl chloride. With DMF as solvent and pyridine as base, the reaction effect was the best.
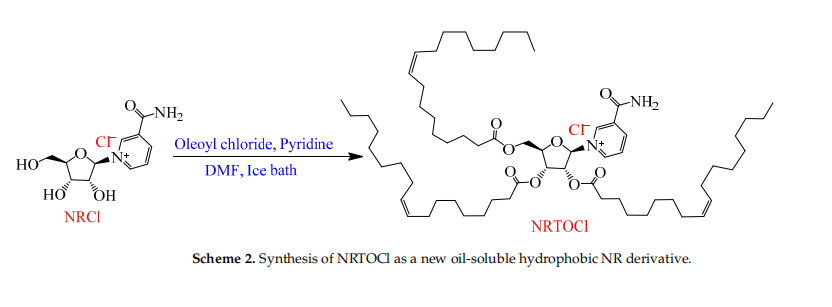
Chemical properties of NRTOCl
NRTOCl is a quaternary ammonium salt, but it is insoluble in water due to the three hydrophobic arms of the oleate. Using EtOH as cosolvent, NRTOCl nanoparticles with an average size of 192 nm and layered structure were dispersed in water. The stability of NRCl and NRTOCl in water for 28 days at 35℃ proved that the stability of NRTO was more than 88 times that of NRCl. In contrast to NRCl, NRTOCl is readily dissolved in canola oil, corn oil, and MCT oil at room temperature.
Reference:
[1] AMIN ZAREI. Synthesis, Stability, and Bioavailability of Nicotinamide Riboside Trioleate Chloride.[J]. Nutrients, 2021. DOI:10.3390/nu14010113.
- Related articles
- Related Qustion
- What are the benefits of Nicotinamide riboside chloride supplement? What is the appropriate daily dosage? May 15, 2025
Nicotinamide riboside chloride (NRCl) is an FDA-approved nutritional supplement that can be used to increase NAD+ levels.
- Niacin vs Niacinamide vs Nicotinamide Riboside: what's the difference? Mar 21, 2024
Nicotinic acid, nicotinamide and nicotinamide riboside are the vitamin B3 precursors of nicotinamide adenine dinucleotide (NAD) in the human diet.
- Nicotinamide riboside chloride: biological activities and preclinical studies Jan 24, 2024
Nicotinamide riboside chloride increases NAD+ levels, has similar toxicity to nicotinamide, and shows potential benefits in metabolism, liver health, and exercise performance.
Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic.....
Nov 16,2023Organic reagentsThe Lewis structure of the nitrite ion is made up of a central atom, nitrogen (N), and three oxygen atoms (O), the outer atoms.....
Nov 16,2023APINicotinamide riboside chloride
23111-00-4You may like
Nicotinamide riboside chloride manufacturers
- Nicotinamide riboside chloride
-
- 2025-12-18
- CAS:23111-00-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Nicotinamide riboside chloride; NR-CL
-

- $0.00 / 1KG
- 2025-12-18
- CAS:23111-00-4
- Min. Order: 1KG
- Purity: ≥98% HPLC
- Supply Ability: 1000KG
- Nicotinamide riboside chloride
-

- $0.00 / 1KG
- 2025-12-18
- CAS:23111-00-4
- Min. Order: 1KG
- Purity: 97%min
- Supply Ability: 100KG