Synthesis and Antiviral activity of PF-07321332
Aug 23,2022
General description
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been causing the COVID-19 pandemic, resulting in several million deaths being reported. Numerous investigations have been carried out to discover a compound that can inhibit the biological activity of the SARS-CoV-2 main protease, which is an enzyme related to the viral replication. Among these, PF-07321332 (Nirmatrelvir) is currently under clinical trials for COVID-19 therapy. Structural comparisons of the hybrid inhibitors with PF-07321332 reveal unconventional F center dot center dot center dot O interactions of PF-07321332 with M-pro which may explain its more favorable enthalpy of binding. BBH-1, BBH-2 and NBH-2 exhibit comparable antiviral properties in vitro relative to PF-07321332, making them good candidates for further design of improved antivirals.
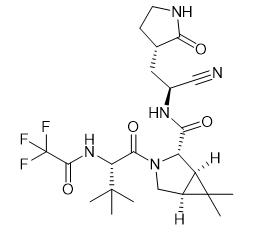
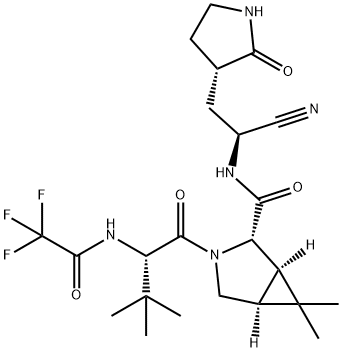
Fig. 1 The structure of PF-07321332.
Synthetic routes
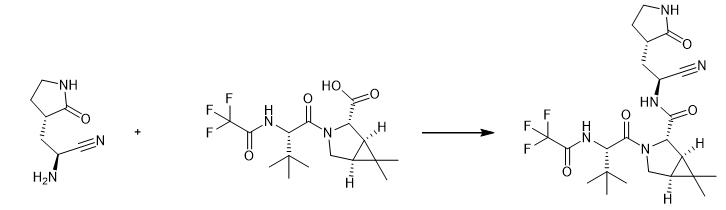
Fig. 2 The synthetic method 1 of PF-07321332.
In a 2 L three-necked flask, add 36.4 g (0.1 mol) of solid material SM1, dissolve with 360ml butanone, then add 19.9 g Intermediate C (0.105 mol), 8.3g HOPO (0.075 mol) and stir for 10min, then add 38.7 g DIPEA (0.3 mol), stirred for 10min, then added 26.6 g EDCI (0.15 mol), reacted at 20-25°C for 16-18 h, the raw material point basically disappeared in TLC, and phosphoric acid aqueous solution (85%, 0.15 mol, 47.5 g was added to the reaction solution) ) and 360 ml of saturated sodium chloride solution, stirred for 30 min, left to stand for stratification, let go of the lower water layer, add 360 ml of sodium chloride solution and 360 ml of isopropyl acetate solution to the organic layer, extract and separate liquids, and concentrate the organic layer to obtain 45.6 g of crude product, the yield is 91.3%. In the there-necked flask of 2 L, add crude product (50 g, 0.1 mol), 500 ml isopropyl acetate, be heated to 65-70°C after solids are all dissolved, under this temperature condition, add the n-heptane of 750 ml , heat preservation and stirring 6-7 h, separate out a large amount of solid, be cooled to room temperature, filter, filter cake is washed with the organic solvent F of 300 ml (wherein, organic solvent F is that isopropyl acetate and n-heptane are mixed by volume ratio of 1:1 mixed solution), and dried to obtain 46.2 g of pure product with a yield of 92.3% [1].
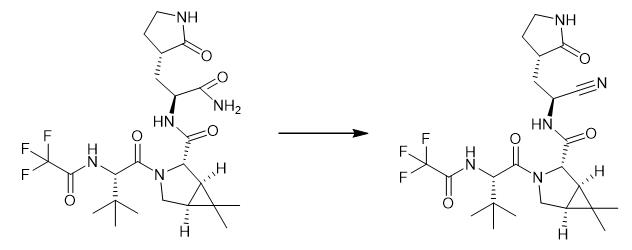
Fig. 3 The synthetic method 2 of PF-07321332.
The compound (51.45 kg, 2.8 mol) was dissolved in DCM (29 L), under nitrogen blanket, triethylamine (Et3N, 1.70 kg, 16.8 mol) was added. The reaction solution is cooled to-10-0°C, and trifluoroacetic anhydride (TFAA, 1.76 kg, 8.4 mol) is slowly dropped in the reaction solution, and the temperature in the control reaction system is lower than 5 °C. After the completion of the dropwise addition, stirring was carried out at 0 °C for 30 min, and the reaction progress was monitored by high performance liquid chromatography (HPLC). After the reaction was completed, the reaction solution was poured into a 1N ammonium chloride solution (12 L), stirred evenly, and the reaction solution was left standstill for stratification, and the organic phase was separated. The organic phase was washed with saturated sodium chloride solution (10 L), dried by adding anhydrous sodium sulfate, the organic phase was separated and evaporated to dryness. With ethyl acetate, n-heptane stirring and crystallization to give white solid compound 6 (1.18 mg, 70% 1 (1:84%) [2].
Antiviral
The current pandemic of multiple variants has created an urgent need for effective inhibitors of SARS-CoV-2 to complement vaccine strategies. PF-07321332, developed by Pfizer, is the first orally administered coronavirus-specific main protease inhibitor approved by the FDA. The high mutation rate of COVID-19 and the prevalence of multiple variants strongly support the need for pharmacological options to complement vaccine strategies. One region that appears highly conserved among different genera of coronaviruses is the substrate-binding site of the main protease (M-pro or 3CL(pro)), making it an attractive target for the development of broad-spectrum drugs for multiple coronaviruses. PF-07321332, developed by Pfizer, is the first orally administered inhibitor targeting the main protease of SARS-CoV-2, which also has shown potency against other coronaviruses. Here, we report three crystal structures of the main protease of SARS-CoV-2, SARS-CoV, and Middle East respiratory syndrome (MERS)-CoV bound to the inhibitor PF-07321332. The structures reveal a ligand-binding site that is conserved among SARS-CoV-2, SARS-CoV, and MERS-CoV, providing insights into the mechanism of inhibition of viral replication. The long and narrow cavity in the cleft between domains I and II of the main protease harbors multiple inhibitor-binding sites, where PF-07321332 occupies subsites S1, S2, and S4 and appears more restricted than other inhibitors. A detailed analysis of these structures illuminated key structural determinants essential for inhibition and elucidated the binding mode of action of the main proteases from different coronaviruses. Given the importance of the main protease for the treatment of SARS-CoV-2 infection, insights derived from this study should accelerate the design of safer and more effective antivirals. IMPORTANCE The current pandemic of multiple variants has created an urgent need for effective inhibitors of SARS-CoV-2 to complement vaccine strategies. PF-07321332, developed by Pfizer, is the first orally administered coronavirus-specific main protease inhibitor approved by the FDA. We solved the crystal structures of the main protease of SARS-CoV-2, SARS-CoV, and MERS-CoV that bound to the PF-07321332, suggesting PF-07321332 is a broad-spectrum inhibitor for coronaviruses. Structures of the main protease inhibitor complexes present an opportunity to discover safer and more effective inhibitors for COVID-19 [3].
The chemical structure of PF-07321332, the first orally available Covid-19 clinical candidate, has recently been revealed by Pfizer. No information has been provided about the interaction pattern between PF-07321332 and its biomolecular counterpart, the SARS-CoV-2 main protease (M-pro). In the present work, we exploited Supervised Molecular Dynamics (SuMD) simulations to elucidate the key features that characterise the interaction between this drug candidate and the protease, emphasising similarities and differences with other structurally related inhibitors such as Boceprevir and PF-07304814. The structural insights provided by SuMD will hopefully be able to inspire the rational discovery of other potent and selective protease inhibitors [4].
The novel coronavirus disease, caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), rapidly spreading around the world, poses a major threat to the global public health. Ahmad et al. demonstrated the binding mechanism of PF-07321332, alpha-ketoamide, lopinavir, and ritonavir to the coronavirus 3-chymotrypsin-like-protease (3CLpro) by means of docking and molecular dynamic (MD) simulations. The analysis of MD trajectories of 3CLpro with PF-07321332, alpha-ketoamide, lopinavir, and ritonavir revealed that 3CLpro-PF-07321332 and 3CLpro-alpha-ketoamide compl
- Related articles
- Related Qustion
- How to synthesise Nirmatrelvir on a large scale? Jan 12, 2024
Nirmatrelvir is synthesised using saponification of bicycloproline ester.
Paxlovid
2628280-40-8You may like
Paxlovid manufacturers
- Nirmatrelvir
-

- $0.00 / 10g
- 2025-12-18
- CAS:2628280-40-8
- Min. Order: 10g
- Purity: 99%-102%
- Supply Ability: 10kgs
- PF-07321332
-

- $0.00 / 1kg
- 2025-12-16
- CAS:2628280-40-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 2500kg
- Paxlovid
-

- $100.00 / 50kg
- 2025-12-16
- CAS:2628280-40-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton