Synthesis of N-Methylphthalimide and its derivatives
Sep 19,2025
Introduction
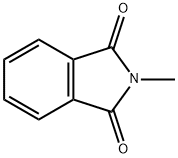
N-methylphthalimide, also konwn as 2-methyl-1h-isoindole-1,3 (2H) -dione, is an important organic chemical intermediate with a wide range of applications. Its molar mass is 161.16g/mol and melting point is 134~135℃. Pure product is milky white acicular crystal.(Figure 1) It is an important raw material for a series of fine chemicals, mainly used in the synthesis of 4-amino-N-methylphthalimide and other resins and pharmaceutical products. 4-Amino-N-methylphthalimide is an important intermediate for the synthesis of isoluminol derivatives. The molecular structure of 4-nitro-N-methylphthalimide is aiso an important intermediate for the synthesis of various polyimide monomer dianhydride.[1]

Synthetical method of N-methylphthalimide
Report 1:
Synthesis of N-methylphthalimide started from phthalic anhydride and aqueous methylamine. The end product was identified by means of FTIR. Effect of feed ratio,reaction tamperature and reaction time was discussed. The optimal reaction condition was obtained with molar ratio phthalic anhydride and methylamine 1:2,reaction temperature 150℃, reaction time 4h. The yield of N-methylphthalimide was 85.5%.[1]
Report 2:
N-methylphthalimide(NMP) was synthesized from phthalic anhydride(PA) and dimethyl carbonate(DMC) with N, N-dimethylformamide (DMF) as solvent and tetrabutylamonium bromide(TBBA) as phase transfer catalyst (PTC), Phthalic anhydride was transformed to phthalic anhydride potassium (PAP) with potassium hydroxide, and then phthalic anhydride potassium was methylated with dimethyl carbonate,.When the molar ratio of n(PAP) : n(DMC) was 1:2, the amount of tetrabutylamonium biomide was 4% of n(PAP), reaction temperature was 120 ℃ , reaction time was 4 h,and appropriate quantity solvent, the yield of N-methylphthalimide reached 70%. The final product was identified comparing with standard N-methylphthalimide sample.[2]
Synthesis of N-methylphthalimide-based derivatives
Palladium N-heterocyclic carbene complexes
A series of novel benzimidazolium salts (1–4) and their pyridine enhanced precatalyst preparation stabilization and initiation(PEPPSI) themed palladium N-heterocyclic carbene complexes [PdCl2(NHC)(Py)] (5–8), where NHC = 1-(N-methylphthalimide)-3-alkylbenzimidazolin-2-ylidene and Py = 3-chloropyridine, were synthesized and characterized by means of 1H and 13C{1H} NMR, UV–vis (for 5–8), ESI-FTICR-MS (for 2, 4, 6–8) and FTIR spectroscopic methods and elemental analysis. The synthesized compounds were tested in Suzuki–Miyaura cross-coupling (for 1–8) and arylation (for 5–8) reactions. As catalysts,they demonstrated a highly efficient route for the formation of asymmetric biaryl compounds even though they were used in verylow loading. For example, all compounds displayed good catalytic activity for the C–C bond formation of 4-tert-butylphenylboronic acid with 4-chlorotoluene.[3]
Benzophenone (BP)-N-methylphthalimide (MePI) dyads
In the present paper, we synthesized a series of benzophenone (BP)-N-methylphthalimide (MePI) dyads (Cn, n = 3, 6, and 9, where n denotes the number of methylene in the linker) and investigated the photochemical properties and intramolecular triplet-triplet energy transfer from BP(T1) to MePI. Formation of two different intramolecular complexes was found, that is, a ground-state complex and a singlet exciplex. The formation of the triplet-equilibrium between MeBP and MePI was observed. The triplet-equilibrium constant (1.0 and 1.1 for C6 and C9, respectively) and forward ((3.8±1.3) x 107 and (3.9±1.2) x 107 s-1 for C6 and C9, respectively) and back ((3.8±1.3) x 107 and (3.6±1.2) x 107 s-1 for C6 and C9, respectively) energy transfer rates were estimated from the result of transient absorption measurements. From the van't Hoff plots, enthalpy and entropy change for the equilibrium formation were estimated.[4]
N-methylphthalimide-based high-washable azo disperse dyes
Cellulose diacetate fibers were prepared from cellulosic biomass with high α-cellulose contents such as purified cotton linters and wood pulps. Cellulose diacetate fibers are sensitive to alkaline solution, which causes hydrolysis of the acetate ester to hydroxyl groups, especially at high temperatures. Thus, the low alkali-resistance of cellulose acetate fibers makes it difficult to achieve high wash fastness by restricting the application of intense after-treatment, such as reduction clearing. A series of N-methylphthalimide-based high-washable azo disperse dyes were synthesized and their dyeing and fastness properties on cellulose diacetate fabrics were investigated. From the overall results obtained in this study, N-methylphthalimidylazo disperse dyes are expected to be a desirable alternative to high value-added dyes that can be used for high color fastness dyeing of cellulose diacetate with a minimal discharge of wastewater during washing process.[5]
References
[1] Guan C,et al. New synthesis method for N-methylphthalmide[J].Journal of Nanjing Tech University(Natural Science Edition),2007,(04):77-79.
[2] Zhou YM,et al.New Process for synthesis of N-methylphthalimide [J].Speciality Petrochemicals,2008,25(04):4-6.
[3]Akkoç S, Gök Y, İlhan İÖ, Kayser V. N-Methylphthalimide-substituted benzimidazolium salts and PEPPSI Pd-NHC complexes: synthesis, characterization and catalytic activity in carbon-carbon bond-forming reactions. Beilstein J Org Chem. 2016;12:81-88. Published 2016 Jan 15. doi:10.3762/bjoc.12.9
[4]Sakamoto M, Kim SS, Fujitsuka M, Majima T. Reversible intramolecular triplet-triplet energy transfer in benzophenone-N-methylphthalimide dyad. J Phys Chem A. 2008;112(7):1403-1407. doi:10.1021/jp075885g
[5]Yoon S, Kim H, Lee E, et al. Synthesis and Application of N-methylphthalimidylazo Disperse Dyes to Cellulose Diacetate for High Wash Fastness. Materials (Basel). 2020;13(21):4924. Published 2020 Nov 2. doi:10.3390/ma13214924
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering6-Bromo-2-naphthol enhances room-temperature phosphorescence (RTP) in Pluronic-SDS micelles and synergizes with polymyxin to lower MIC.....
Sep 19,2025APIN-Methylphthalimide
550-44-7You may like
N-Methylphthalimide manufacturers
- N-methylphthalimide
-

- $0.00 / 25kgs/bag
- 2025-12-05
- CAS:550-44-7
- Min. Order: 25kgs/bag
- Purity: 99%
- Supply Ability: 10 tons
- N-methylphthalimide
-

- $0.00 / 1kg
- 2025-09-16
- CAS:550-44-7
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 500 tons
- N-Methylphthalimide
-

- $45.00 / 1kg
- 2025-05-23
- CAS:550-44-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons