tert-Pentanol: Properties, Process for manufacture and Uses
Mar 4,2024
Properties of tert-pentanol
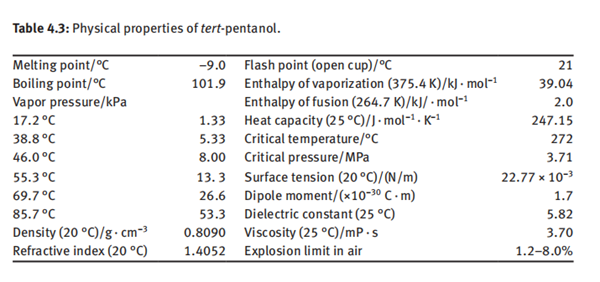
tert-Pentanol, also known as 2-methyl-2-butanol, tert-pentyl alcohol, tert-amyl alcohol, dimethyl ethyl carbinol, ethyl dimethyl carbinol and 1,1-dimethyl-1-propanol, is a derivative of methylbutynol. tert-Pentanol is a colorless, transparent and volatile liquid at room temperature with a special pungent odor. It is partially soluble in water. The solubility in water is 14% (mass fraction) at 30°C, and it is miscible with ethanol, ether, benzene, chloroform, glycerin and the like. tert-Pentanol and water form an azeotrope at composition of 72.5% tert-pentanol and 27.5% water, having an azeotropic point of 87.35°C. It can also form azeotropes with various organic solvents such as benzene, toluene, cyclohexane, methylcyclohexane, carbon tetrachloride, heptane, octane, propyl acetate and the like. The main physical properties of tert-pentanol are listed in Table 4.3.
When tert-pentanol is exposed to air for a long time, it will be oxidized to high-boiling substances and the color turns yellow. Therefore, it is necessary to purge the container with a dry inert gas such as nitrogen to remove air and to maintain a protective atmosphere throughout the storage period. Since tert-pentanol is an organic solvent with excellent dissolving performance, it cannot be stored in a container of plastic and rubber. However, the storage and transportation container for tert-pentanol can be made of carbon steel, stainless steel or aluminum.
Process for manufacture of tert-pentanol
Industrial production methods of tert-pentanol include acetylene–acetone route and isopentene hydration route. The acetylene–acetone route uses acetylene from natural gas or calcium carbide as a raw material, while the isopentene hydration route uses isopentene from petroleum as a raw material. In China, tert-pentanol is produced by acetylene–acetone route.
Acetylene reacts with acetone to form methylbutynol. Hydrogenation of methylbutynol yields tert-pentanol:

Acetylene and ammonia were mixed in a certain ratio, and the mixture was pressurized to a certain pressure by a compressor and condensed into a liquid by a condenser. Acetone is pumped into the reactor simultaneously with the condensate of the gas mixture according to a certain proportion. The catalyst sodium hydroxide was added to the reactor from the top and was in countercurrent contact with the reactants. After reaction, the reaction mixture was neutralized by HCl. The unreacted acetylene and ammonia were evaporated by a flash evaporator and recycled.
Acetone was recovered from the reaction solution after flash evaporation by an acetone recovery tower for reuse. The reaction solution was then subjected to fractional distillation for obtaining an azeotrope of methylbutynol and water.
The methylbutynol–water azeotrope was introduced into the first-stage hydrogenation reactor, and flowed down with hydrogen in the same direction through the Pd/α-Al2O3 catalyst layer. The outlet reaction mixture from the reactor was subjected to cooling and gas–liquid separation. Hydrogen was recovered, and the liquid was sent to the secondstage hydrogenation reactor. After the second-stage hydrogenation, the reaction mixture was cooled and separated from hydrogen. Water in the reaction liquid was removed by calcium chloride. Finally, pure tert-pentanol was obtained by fractional distillation.
The process using calcium chloride for water removal has some disadvantages
including long process, large amount of wastewater, low yield of tert-pentanol and
much consumption of calcium chloride. Afterward it has been improved by azeotropic
distillation using cyclohexane as an entrainer. The advantages are as follows: (1)
calcium chloride is eliminated, and the cost of calcium chloride is saved; (2) the yield
of tert-pentanol is increased by about 2.5%, and the cost is reduced; and (3) the environmental pollution caused by calcium chloride wastewater is eliminated.
Technical parameters of operation for tert-pentanol are as follows:
(1) Ethynylation: Volume ratio of acetylene/ammonia in the gas mixture: 1:3; Pressure: 2.0–2.2 MPa; Temperature: 35–40°C; Residence time: 1.0–1.3 h; The yield of methylbutynol: ≥94%.
(2) Hydrogenation: Hydrogen pressure: 0.65–0.70 MPa; Temperature: 35–80°C; Space velocity: 1.5–2.0 h−1; Volume ratio of gas to liquid in feed: 630 to 780:1; Catalyst life: ≥650 h; The conversion of hydrogenation: 100%; The yield of hydrogenation: ≥95%.
The deactivated waste catalyst is regenerated by burning off the polymer with air at a temperature of 450–500°C and then reduced by formaldehyde or hydrazine hydrate solution.
Uses of tert-pentanol
tert-Pentanol is mainly used as a solvent and an intermediate for some pharmaceuticals and fine chemicals. It is used as an intermediate for the synthesis of pesticides such as triazolone, triadimenol and vinyl azoles. tert-Pentanol can also be used for production of indane musk which is a perfume. Furthermore, tert-pentanol is a raw material for the manufacture of tert-amyl peroxypivalate, which is an initiator for the polymerization or copolymerization of ethylene, styrene, butadiene, acrylate and methacrylate. Moreover, tert-pentanol is used as a stabilizer for trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane, 1,2-dichloropropane, and so on. In these solvents, tert-pentanol has the effect of inhibiting their decomposition, volatilization and corrosion.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThe name of the compound (NH4)3PO4 is Ammonium Phosphate. Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula (NH4)3PO4.....
Mar 4,2024Inorganic chemistry2-Methyl-2-butanol
75-85-4You may like
2-Methyl-2-butanol manufacturers
- 2-Methyl-2-butanol
-

- $10.00 / 1KG
- 2025-12-11
- CAS:75-85-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 2-Methyl-2-butanol
-

- $5.00 / 1KG
- 2025-05-26
- CAS:75-85-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- 2-Methyl-2-butanol
-
- $1.50 / 1g
- 2023-07-27
- CAS:75-85-4
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons