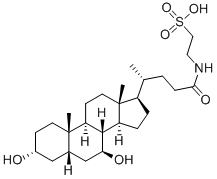
The bile acids——Tauroursodeoxycholic acid
Dec 31,2021
General description
Tauroursodeoxycholic acid is a bile acid taurine conjugate derived from ursoodeoxycholic acid. It has a role as a human metabolite, an anti-inflammatory agent, a neuroprotective agent, an apoptosis inhibitor, a cardioprotective agent and a bone density conservation agent. It derives from an ursodeoxycholic acid. It is a conjugate acid of a tauroursodeoxycholate. Tauroursodeoxycholic acid is the more hydrophilic form of ursodeoxycholic acid, which is the more abundant naturally produced bile acid in humans. Tauroursodeoxycholic acid, on the other hand, is produced abundantly in bears and has been used for centuries as a natural remedy in some Asian countries. It is approved in Italy and Turkey for the treatment of cholesterol gallstones and is an investigational drug in China, Unites States, and Italy. Tauroursodeoxycholic acid is being investigated for use in several conditions such as Primary Biliary Cirrhosis (PBC), insulin resistance, amyloidosis, Cystic Fibrosis, Cholestasis, and Amyotrophic Lateral Sclerosis. The only completed clinical trial thus far is a phase III clinical trial comparing tauroursodeoxycholic acid and ursofalk in PBC adult patients, but as of June 2013 no results of this trial have been published.
Bile plays an essential physiological role in the human body. It is produced by hepatocytes to take part in fat digestion. Abnormalities in bile secretion can result in digestion problems and serious hepatobiliary diseases. It is believed that exogenous administration of bile components can be a reasonable therapeutic approach improving outcomes of cholestatic liver diseases. One of the bile components with beneficial effects in alleviating liver damage in a setting of cholestasis is UDCA. UDCA was first synthesized in 1950 and successfully introduced into clinical treatment of liver diseases [43,46]. Benefits of using UDCA in a wide range of hepatobiliary disorders such as primary sclerosing cholangitis (PSC), pediatric cholestatic disorders, primary biliary cholangitis (PBC), and drug-induced cholestasis, have been evaluated in a series of clinical studies. Although in patients with PBC, UDCA has been demonstrated to significantly improve liver function, its use in PSC remains more controversial. Not only is this therapy barely effective in reducing liver enzymes, but also there are no positive effects on long-term survival and side effects of high doses of UDCA are serious enough not to suggest its routine use in PSC. Bile acids are produced in the liver and excreted into the intestine, where their main function is to participate in lipid digestion. Ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) have shown antiapoptotic, anti-inflammatory, and antioxidant effects in various models of neurodegenerative diseases. However, little is known about signaling pathways and molecular mechanisms through which these bile acids act as neuroprotectors, delaying translation to the clinical setting.
Application and pharmacology
Tauroursodeoxycholic acid (TUDCA) is a naturally occurring hydrophilic bile acid that has been used for centuries in Chinese medicine. Chemically, TUDCA is a taurine conjugate of ursodeoxycholic acid (UDCA), which in contemporary pharmacology is approved by Food and Drug Administration (FDA) for treatment of primary biliary cholangitis. Interestingly, numerous recent studies demonstrate that mechanisms of TUDCA functioning extend beyond hepatobiliary disorders. Thus, TUDCA has been demonstrated to display potential therapeutic benefits in various models of many diseases such as diabetes, obesity, and neurodegenerative diseases, mostly due to its cytoprotective effect. The mechanisms underlying this cytoprotective activity have been mainly attributed to alleviation of endoplasmic reticulum (ER) stress and stabilization of the unfolded protein response (UPR), which contributed to naming TUDCA as a chemical chaperone. Apart from that, TUDCA has also been found to reduce oxidative stress, suppress apoptosis, and decrease inflammation in many in-vitro and in-vivo models of various diseases. The latest research suggests that TUDCA can also play a role as an epigenetic modulator and act as therapeutic agent in certain types of cancer[5]. Nevertheless, despite the massive amount of evidence demonstrating positive effects of TUDCA in pre-clinical studies, there are certain limitations restraining its wide use in patients.[1]
Synthesis
1.Biosynthesis:UDCA is a secondary bile acid that can be produced exclusively by intestinal microbiota. Human liver is able to synthesize from cholesterol only primary bile acids such as chenodeoxycholic acid (CDCA) and cholic acid, which are further conjugated with glycine or taurine and secreted in bile in a form of taurocholic acid, glycocholic acid, glycochenodeoxycholic acid, and taurochenodeoxycholic acid. Further processing of primary bile acids is performed by gut bacteria and finally results in the formation of secondary bile acids such as UDCA [3,5]. The main pathway of TUDCA biosynthesis includes the conversion of cholesterol to 7α-hydroxycholesterol by microsomal cholesterol 7α-hydroxylase (CYP7A1). Next, 7α-hydroxycholesterol is converted to 7α-hydroxy-4-cholestene-3-one by 3β-hydroxy-Δ5-C27-steroid dehydroxylase (3β-HSD, HSD3B7). This is followed by the series of reactions including steroid side chain cleavage catalyzed by the mitochondrial sterol 27-hydroxylase (CYP27A1) to form chenodeoxycholic acid [4]. Alternatively, cholesterol might be transformed to 27-hydroxycholesterol by CYP27A1, then hydroxylated to 3β-7α-dihydroxy-5-cholestenoic acid by oxysterol 7α- hydroxylase (CYP7B1), and finally converted by CYP27A1 to CDCA [4]. CDCA and its conjugates are then secreted with bile into the large intestine where gut microbiota transforms it into UDCA. Finally, UDCA returns to the liver with enterohepatic circulation and is there conjugated with taurine to form TUDCA. [2]
2.Chemical synthesis: UDCA was used to form mixed anhydride with alkyl chloroformate or aromatic ester, and then reacted directly with taurine to prepare TUDCA. Add ursodeoxycholic acid into the reactor, add acetone at room temperature to dissolve, stir and dissolve. After the temperature drops to 10 ℃, slowly add triethylamine. After the temperature drops to 0 ℃, add ethyl chloroformate, stir and react, gradually crystallize, filter, and clean the filter cake with acetone. The product of the above step is added into the soda solution of taurine, stirred for 1h, and then concentrated hydrochloric acid is added to obtain the crude product of TUDCA. Stir fully, add acetone for crystallization, add water in the reactor, heat to 80 ℃, add the crude product in the previous step, add an appropriate amount of water, heat to 80 ℃, stir for 1h, cool to 0 ~ 5 ℃, add ether, stir for 48h for crystallization and purification.[3]
Figure the systhesis route of Tauroursodeoxycholic acid
Compared with the phenol ester method, this method omits the product generated after the mixed anhydride forms the ester. The advantage is that the use of toxic p-nitrophenol can be omitted, the reaction temperature control meets the equipment conditions, the cost is low, the process route is short, and the yield is as high as 78% [13]. It is suitable for industrial scale production.[3,4]
History
For centuries, traditional Chinese medicine has valued animal bile for its use in pharmacological and clinical applications. Bile acids are natural products and fundamental components of bile. Tauroursodeoxycholic acid (TUDCA) is a taurine conjugate of ursodeoxycholic acid (UDCA). Based on its choleretic activity and capability of protecting hepatocytes, UDCA has been approved by FDA (US Food and Drug Administration) for treatment of primary biliary cholangitis (previously primary biliary cihrrosis). The advantage of bile acids in therapeutic use is that they might be administrated via oral, subcutaneous, and intravenous routes of application. Furthermore, in most cases they present rather low toxicity to the organism and are able to cross the blood-brain barrier. These favorable characteristics of TUDCA are the main reason why it has been studied as a potential therapeutic agent in a broad spectrum of diseases. Taurine ursodeoxycholic acid capsule was successfully developed and approved for production by the Italian best pharmaceutical factory in one year. It was certified by the U.S. production process patent in. It was first listed in Italy in. It was approved for sale in China under the trade name taoluoteding in. The latest registration certificate number is and the specification is box. It is mainly used for the treatment of cholesterol stones in gallbladder or bile duct clinically.
Toxicity
TUDCA’s ability to inhibit apoptosis and promote survivalpathways may prove to be an effective therapy for TBI. Toassess the efficacy of TUDCA for TBI, it should first be evaluated in the laboratory setting .Dose escalation as well as time and period of intervention must be carefully studied. Route of administration such as IV or IP can yield a higher bioavailability as compared to oral ingestion of TUDCA, which should be considered for dose escalation studies. Relevant histological and behavioral studies can shed light on how TUDCA can ameliorate the symptoms of TBI. Based on the outcomes of preclinical studies and lessons learned, future clinical studies need to focus on the safety and efficacy of treating TBI patients with TUDCA, appropriate dosing, time of injury on set to therapy, and duration of therapy, along with assessing long-term outcomes in patients who have undergone treatment with TUDCA following their injury.[5]
Reference
1.Gronbeck K. R., Rodrigues C. M. P. & Mahmoudi J. et al., "Application of Tauroursodeoxycholic Acid for Treatment of Neurological and Non-neurological Diseases: Is There a Potential for Treating Traumatic Brain Injury?" Neurocritical Care, Vol.25, No.1(2016), pp.153-166.
2.Daruich A., Picard E. & Boatright J. H. et al., "Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease," Mol Vis, Vol.25(2019), pp.610-624.
3.Chai Chuanke: synthetic process and industrial design of tauroursodeoxycholic acid: East China University of technology, 2012
4.Wang Peng, Zhu Rongju: progress in synthesis of taurine ursodeoxycholic acid, amino acids and biological resources, 2013, No. 02, pp. 46-50
5.Kusaczuk M., "Tauroursodeoxycholate—Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives," Cells, Vol.8, No.12(2019), p.1471.
- Related articles
- Related Qustion
- Tauroursodeoxycholic Acid: Characteristics and Therapeutic Effect on Alzheimer's Disease Jul 2, 2024
Tauroursodeoxycholic acid shows promise for treating Alzheimer's by improving cognition via neuroprotective mechanisms, warranting further clinical validation for human use.
- Tauroursodeoxycholic Acid: Exploring its Vital Role in Various Biological Processes May 29, 2023
Tauroursodeoxycholic acid is a bile acid derived from ursodeoxycholic acid. It is naturally produced in the body through a series of enzymatic reactions in the liver.
Sodium nitrite appears as a yellowish white crystalline solid. Noncombustible but will accelerate the burning of combustible material. If large quantities are involved in a fire or if the combustible....
Dec 31,2021Inorganic saltsEpitalon is a synthetic peptide, telomerase activator, and putative anti-aging drug developed by the St. Petersburg Institute of Bioregulation and Gerontology,which was identified as the putative active component of a bovine pineal gland ex....
Dec 31,2021Biochemical EngineeringTauroursodeoxycholic acid
14605-22-2You may like
Tauroursodeoxycholic acid manufacturers
- Tauroursodeoxycholic acid
-

- $165.00 / 25g
- 2025-12-15
- CAS:14605-22-2
- Min. Order: 25g
- Purity: 0.98
- Supply Ability: 25kg
- Tauroursodeoxycholate
-
- $50.00 / 50mg
- 2025-12-15
- CAS:14605-22-2
- Min. Order:
- Purity: 99.91%
- Supply Ability: 10g
- Tauroursodeoxycholic acid
-

- $0.00 / 1KG
- 2025-12-15
- CAS:14605-22-2
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 1000KG