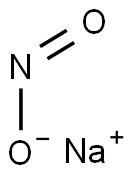Sodium Nitrite—An All-Rounder with Good and Evil
Dec 31,2021
General description
Sodium nitrite appears as a yellowish white crystalline solid. Noncombustible but will accelerate the burning of combustible material. If large quantities are involved in a fire or if the combustible material is finely divided, an explosion may result. If contaminated by ammonium compounds, spontaneous decomposition can occur and the resulting heat may ignite surrounding combustible material. Prolonged exposure heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. Used as a food preservative, and to make other chemicals.
Application and pharmacology
Sodium nitrite is the most commonly used food additive in the production of meat products. It has moderately strong toxicity. It has important applications in chemical, pharmaceutical, textile, machinery and other industries. Sodium nitrite is an inorganic sodium salt having nitrite as the counterion. Used as a food preservative and antidote to cyanide poisoning. It has a role as an antimicrobial food preservative, an antihypertensive agent, a food antioxidant, a poison and an antidote to cyanide poisoning. It is a nitrite salt and an inorganic sodium salt.
Synthesis
Made from sodium nitrite. The chemical formula is NaNO2. Nitrous acid only exists in dilute aqueous solution. It is a weak acid, unstable and easy to decompose into NO2 and No. the following disproportionation reaction can also occur: 3HNO2 = = = HNO3 + 2NO ↑ + H2O. The oxidation number of nitrogen atom in nitrous acid is + 3, which is an intermediate oxidation state. Therefore, nitrous acid has both oxygenation and reduction, and the oxidation is more prominent than reduction. For example, it can oxidize i-ions into elemental iodine in aqueous solution: dissolve the mixture of nitrogen dioxide and nitrogen oxide in water close to zero, that is, the aqueous solution of nitrous acid: NO2 + NO + H2O = = = 2hno2. Add acid to nitrite solution to obtain nitrous acid solution: NaNO2 + HCl = = = HNO2 + NaCl. Nitrous acid is used in organic synthesis in industry, Azo dyes are prepared by converting amines into diazo compounds. Nitrous acid can be divided into CIS and trans, and trans nitrous acid is more stable than CIS nitrous acid.
Figure the systhesis route of Sodium nitrite
It is a by-product of nitric acid production. The tail gas discharged from the industrial production of nitric acid contains a large amount of nitric oxide and nitrogen dioxide. The Neutralization Solution between it and sodium nitrate can be obtained by absorbing these tail gases with soda ash (Na2CO3) or caustic soda (NaOH) solution. After evaporation, crystallization and other operations, using their different solubility in water, the finished products with high quality, high price and low cost can be obtained after separation.[2]
History
From the source, N-nitroso compounds mainly come from food and Precursors of nitroso compounds and their synthesis in humans. Taking food sources as an example, vegetables and fruits contain certain nitrite, nitrate and amine substances. When these substances are stored and processed, rotted or eaten excessively, a series of reactions will occur to produce nitrosamine and convert nitrite. In livestock, poultry and fish products, amine compounds will appear after cooking. When they react with nitrite, they will convert nitrosamines. It contains a small amount of nitrosamine in dairy products and has a certain volatility. Due to the presence of Bacillus subtilis, nitrate will become nitrite under reduction reaction. In addition, there will be some inedible nitrite and nitrate in the boiled water, which will lead to the appearance of nitrite when cooking food. Taking the synthesis in human body as an example, when pH < 3, it is easy to synthesize nitrite amine in human gastric juice, and the content of nitrate to nitrite is increased by 6 times under the reduction reaction. Moreover, human nitric oxide synthase exists. When it is transformed with arginine, it can produce citrulline and nitric oxide, and then release nitrite through a series of reactions. In particular, nitrate in saliva accounts for 5% to 8% of the total intake in the human body, which can be converted into nitrite.
Toxicity
Sodium nitrite solution appears as a clear colorless to yellow solution. Harmful to the environment and somewhat toxic. Used as a preservative, and to make other chemicals. For the residue of sodium nitrite in livestock and poultry products in canned meat and meat mouth, the control requirements are calculated with NaNO2, and the canned meat is controlled within 50 mg / kg and the meat products are controlled within 30 mg / kg. At present, the maximum dosage of sodium nitrate is generally controlled within 0.5g per kilogram, and the residue is calculated based on sodium nitrite. For another example, sodium nitrate is widely used in the anti-corrosion treatment of cheese. However, under the current standard, whether it is used in combination with potassium nitrate or independently, its maximum dosage needs to be controlled within 0.5g per kilogram. Therefore, it is suggested that in the formulation of management standards, based on the previous management standards, scientific and reasonable selection should be made to comprehensively monitor the content of N-nitroso compounds in food in real time.[3]
The LD50 value of toxic substances taken orally is 200 mg/kg−1, the LD50 value of highly toxic substances taken orally is 50 mg/kg−1, while the LD50 value of sodium nitrite is only 85 mg/kg−1 (this value is for rats taken orally). Therefore, it only has poisons, but it does not belong to severe poisons. However, in the food industry, we can't easily give up our vigilance against it. Doctors also said that if people eat it 0.3-0.5 g at a time, it will cause poisoning. When it exceeds 3 g, they may even die directly.
Reference
1.Wei Liang Jin,Sodium nitrite: a generalist who is both good and evil.
2.Li Xiaoli, Analysis of hazards and preventive measures of N-nitroso compounds.
- Related articles
- Related Qustion
- What are the health risks of Sodium nitrite overdose? Apr 23, 2024
Sodium nitrite is a preservative, and ingestion of foods containing excessive amounts of sodium nitrite can also result in acute poisoning or death.
- Sodium nitrite: Toxicity, Application and Preparation Apr 24, 2023
Sodium nitrite is a salt and an inorganic compound with the chemical formula NaNO2. Sodium nitrite is commonly used as a food preservative, particularly for cured meats.
Phosphoric acid appears as a clear colorless liquid or transparent crystalline solid. The pure solid melts at 42.35°C and has a density of 1.834 g / cm3. Liquid is usually an 85% aqueous solution.....
Dec 31,2021Inorganic acid EstersTauroursodeoxycholic acid is a bile acid taurine conjugate derived from ursoodeoxycholic acid. It has a role as a human metabolite, an anti-inflammatory agent, a neuroprotective agent, an apoptosis in....
Dec 31,2021Pharmaceutical intermediatesSodium nitrite
7632-00-0You may like
- Sodium nitrite
-

- $10.00 / 1KG
- 2025-12-11
- CAS:7632-00-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Sodium Nitrite
-

- $9.00 / 1KG
- 2025-05-26
- CAS:7632-00-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 ton
- Sodium nitrite
-

- $660.00 / 25tons
- 2024-04-30
- CAS:7632-00-0
- Min. Order: 26tons
- Purity: 98%
- Supply Ability: 2000tons