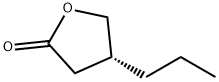
The exploration of synthesis route of (R)-4-Propyldihydrofuran-2(3H)-one
Oct 18,2023
Background
Epilepsy is a disorder of the brain characterized by repeated seizures. A seizure is usually defined as a sudden alteration of behavior due to a temporary change in the electrical functioning of the brain. As a chemical analog of levetiracetam, Brivaracetam is used to help control partial onset seizures in the treatment of epilepsy.
Benoit M. Kenda et al. describe the preparation of 2-oxo-pyrrolidine with racemic 4-n-propyl-dihydrofuran-2(3H)-one-based method. Due to this starting material, the obtained product is a pair of diastereomers, since the isomers are similar in nature to the principal components and are difficult to pass conventional weights. The crystallization method is removed, and therefore, it must be separated by chiral column preparation to obtain a qualified product. Some researchers have reported that Using optically pure (R)-4-Propyldihydrofuran-2(3H)-one as raw material, high chiral purity products can be obtained.
(R) -4-Propyldihydrofuran-2(3H)-one is the key chiral intermediate for the antiepileptic drug Brivaracetam[1]. Consequently, many researchers have been looking for an efficient, simple, and economical method to approach (R)-4-Propyldihydrofuran-2(3H)-one for decades.
Synthetic route 1
Kosugi, H et al. reported a synthetic route of optically pure (R)-4-Propyldihydrofuran-2(3H)-one. The route uses chiral sulfoxide as the starting material and is catalyzed by metal ruthenium to obtain cis olefin. After catalyzed by zinc powder, the reaction with trichloroacetyl chloride closed the ring. Finally, the product was obtained by dechlorination and desulphurization. However, the material is not easy to purchase, and this method uses a precious metal ruthenium catalyst and a highly toxic metal tin catalyst. This method is not suitable for industrial production.
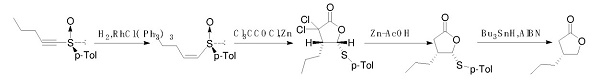
Synthetic route 2
In 2016, Arnaud Schülé et al. reported a synthesis method that uses racemic substituted malonate as a starting material. After enzymatic resolution, the (R) isomer is obtained, followed by reduction and ring closure to obtain a chiral lactone. This route is costly due to the stringent reaction conditions and high-cost enzymatic catalysis[2].
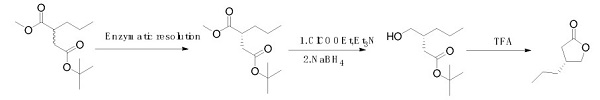
Synthetic route 3
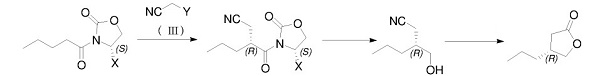
In 2018, Ma et al. Reported a method that uses pure (S)-2-n-pentanoyl-4-substituted oxazole-2-one as a raw material and through the steps of alkylation, reduction, cyano hydrolysis, esterification, etc. to prepare optically pure (R)-4-n-propyl-dihydrofuran-2(3H)-one[3]. The route is as follows:
References
[1] Sun L R, et al. Engineering of an ene-reductase for producing the key intermediate of antiepileptic drug Brivaracetam. Applied Microbiology and Biotechnology, 2023; 107: 1649–1661.
[2] Schülé* A, et al. A Biocatalytic Route to the Novel Antiepileptic Drug Brivaracetam. Org. Process Res. Dev., 2016; 20: 1566–1575.
[3] Ma L, et al. Method for preparing optically pure (r)-4-n-propyl-dihydrofuran-2(3h)-one. WO2018152949A1, 2008; 9: 285–290.
- Related articles
- Related Qustion
- The Rise of (R)-dihydro-4-propyl-2(3H)-furanone in Specialty Chemical Applications May 22, 2024
As the chemical industry continues to evolve, the significance of compounds like (R)-dihydro-4-propyl-2(3H)-furanone becomes increasingly apparent.
- Synthesis and Application of (R)-4-Propyldihydrofuran-2(3H)-one Oct 8, 2022
(R)-4-Propyldihydrofuran-2(3H)-one can be used in laboratory organic synthesis and chemical and pharmaceutical research and development.
It is of great value to develop an effective method to synthesize 2-Butene-1,4-diol which is an important organic compound.....
Oct 17,2023Chemical ReagentsMildronate treats cardiovascular disorders through carnitine regulation and glucose oxidation, but requires careful monitoring for potential side effects.....
Oct 18,2023API(R)-4-Propyldihydrofuran-2(3H)-one
63095-51-2You may like
(R)-4-Propyldihydrofuran-2(3H)-one manufacturers
- Brivaracetam Impurity 19
-

- $0.00 / 10mg
- 2025-09-25
- CAS:63095-51-2
- Min. Order: 10mg
- Purity: 98%
- Supply Ability: 500mg
- (R)-4-Propyldihydrofuran-2(3H)-one
-

- $0.00 / 5KH
- 2024-06-16
- CAS:63095-51-2
- Min. Order: 5KH
- Purity: 99
- Supply Ability: 10Tons
- (R)-4-Propyldihydrofuran-2(3H)-one
-

- $35.00/ kg
- 2023-11-16
- CAS:63095-51-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 Ton