Aluminium Oxide: Properties and Uses
Nov 28,2023
Introduction
Aluminum oxide, often referred to as alumina, is a chemical compound composed of aluminum and oxygen. Its chemical formula is Al2O3. It is one of the most commonly occurring compounds in the Earth's crust and has several important industrial applications. Al2O3 stands as a multifaceted compound that holds a pivotal role in numerous industrial, electronic, and medical applications. Also known as alumina, this compound is formed through the combination of aluminum and oxygen, resulting in a substance characterized by its hardness, thermal stability, and chemical inertness. The unique properties of aluminum oxide have positioned it as an indispensable material across various domains.
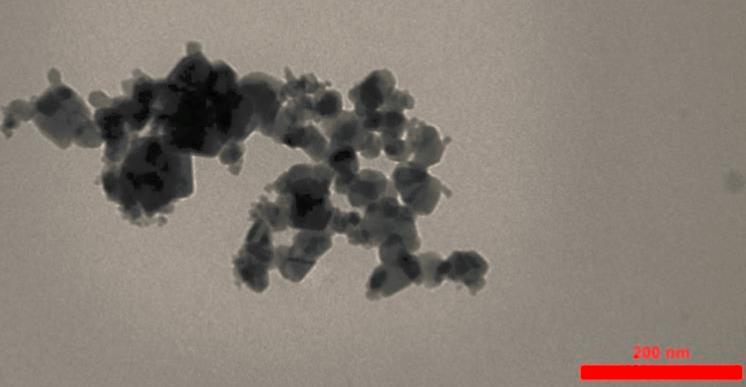
Figure 1 The TEM image of Al2O3 NPs
Application
It is a versatile chemical compound widely found in the Earth's crust, occurring naturally in forms like corundum and as the primary component of bauxite ore, which serves as the fundamental source for aluminum metal production. Its applications span various industries: it is renowned for its hardness and finds use in abrasive materials such as sandpaper and grinding wheels, while also serving as a linchpin in refractory materials for withstanding high temperatures and as a key component in ceramics and porcelain manufacturing. Additionally, it has applications as a catalyst or catalyst support in certain industrial processes and as an electrical insulator. Beyond industrial uses, aluminum oxide has dental and medical applications, including sandblasting and dental cleaning. It comes in various polymorphic forms, such as alpha-Al2O3 and gamma-Al2O3, each with distinct crystal structures and properties. The extraction of aluminum oxide from bauxite ore is crucial in aluminum production, typically following the Bayer process. Furthermore, the refinement of aluminum oxide into high-purity alumina is integral to electronic and advanced materials applications. It should be handled with care, particularly in fine dust or powder form, as inhaling it can be harmful to the respiratory system.
The interaction of Al2O3 NPs with RBCs and Hb to determine the effect of Al2O3 NPs on hemolytic activity and Hb denaturation. The percentage of hemolysis of extracts and direct contact assays triggered by Al2O3 NPs was calculated by determining supernatant Hb concentration at 540 nm. Far-UV CD and Trp/ANS/acrylamide fluorescence spectroscopic methods were used to determine the structural changes of Hb upon interaction with Al2O3 NPs. Theoretical studies were carried out to display the residues involved in the binding site of Hb with Al2O3 nanocluster as well as the structural changes of Hb after interaction. The results showed that the percentage of hemolysis of extract and direct contact assays induced by Al2O3 NPs were 1.16 and 0.46, respectively. Fluorescence spectroscopy revealed that Al2O3 NPs alter the quaternary structure of the protein; however, CD spectroscopy indicated that the secondary structure of Hb remains almost unchanged. Theoretical study displayed that Al2O3 nanocluster interacts with different residues of protein, and Hb tends to be destabilized at the binding site with nanocluster[1].
Synthesis
First, bauxite ore, the primary source of aluminum, is mined and subsequently crushed and ground to a fine powder to facilitate aluminum oxide extraction. The bauxite is then subjected to digestion, a high-pressure process involving a hot concentrated solution of sodium hydroxide (NaOH), which dissolves the aluminum oxide while leaving impurities behind. After digestion, a clarification and separation step removes solid impurities, resulting in a clear sodium aluminate solution. Precipitation occurs by reducing the solution's pH through carbon dioxide (CO2) addition, causing the precipitation of aluminum hydroxide. Following filtration, the precipitated aluminum hydroxide undergoes calcination at high temperatures to yield alumina in the form of a fine white powder. Additional refining processes may be employed to achieve the desired purity for specific applications. The final alumina product is stored, transported, and utilized in various industries. It serves as the primary feedstock for aluminum production, where it undergoes electrolytic reduction in the Hall-Héroult process to produce aluminum metal. Environmental concerns related to waste generated during alumina production, such as red mud residue, emphasize the importance of proper waste management and sustainable production practices.
Safety
Aluminum oxide is generally considered safe for most applications and is not highly toxic. However, there are some safety considerations to keep in mind:
1.Inhalation: Fine dust or powder of aluminum oxide can be harmful if inhaled. Prolonged exposure to high concentrations of airborne aluminum oxide particles can lead to respiratory irritation, lung issues, and potential long-term health concerns. Adequate ventilation and the use of appropriate respiratory protection are important when working with fine particulate forms of aluminum oxide, such as in industrial or abrasive applications.
2.Irritation: Contact with the skin or eyes may cause irritation. It is advisable to wear protective clothing, gloves, and eye protection when handling aluminum oxide, especially if there is a potential for skin or eye contact.
3.Ingestion: While aluminum oxide is generally recognized as safe when ingested in small quantities (it is used as an anti-caking agent in food products), swallowing large amounts of aluminum compounds can lead to health issues. Chronic ingestion of excessive amounts of aluminum has been linked to certain health concerns, including potential neurotoxicity and bone disorders.
4.Environmental Impact: Aluminum oxide is not considered environmentally hazardous, but like other industrial materials, its disposal should be managed properly to minimize its impact on the environment.
It's important to follow safety guidelines and take appropriate precautions when working with aluminum oxide, especially in industrial settings where exposure to fine particles can occur. In many cases, wearing personal protective equipment and working in well-ventilated areas can mitigate potential risks. Additionally, it's crucial to follow specific safety instructions provided by manufacturers and regulatory agencies when using products that contain aluminum oxide. Always consult with relevant safety data sheets (SDS) and adhere to occupational safety and health regulations to ensure safe handling and use of aluminum oxide. a safety assessment of alumina and aluminum hydroxide as used in cosmetics. Alumina functions as an abrasive, absorbent, anticaking agent, bulking agent, and opacifying agent. Aluminum hydroxide functions as a buffering agent, corrosion inhibitor, and pH adjuster. The Food and Drug Administration (FDA) evaluated the safe use of alumina in several medical devices and aluminum hydroxide in over-the-counter drugs, which included a review of human and animal safety data. The Cosmetic Ingredient Review (CIR) Expert Panel considered the FDA evaluations as part of the basis for determining the safety of these ingredients as used in cosmetics. Alumina used in cosmetics is essentially the same as that used in medical devices. This safety assessment does not include metallic or elemental aluminum as a cosmetic ingredient. The CIR Expert Panel concluded that alumina and aluminum hydroxide are safe in the present practices of use and concentration described in safety assessment[2].
Reference
1.Kahbasi S, Samadbin M, Attar F, et al. The effect of aluminum
oxide on red blood cell integrity and hemoglobin structure at
nanoscale[J]. Int J Biol Macromol, 2019,138:800-809.
2.Becker L
C, Boyer I, Bergfeld W F, et al. Safety Assessment of Alumina and
Aluminum Hydroxide as Used in Cosmetics[J]. Int J Toxicol, 2016,35(3
suppl):16S-33S.
- Related articles
- Related Qustion
- The structure of Aluminum oxide May 24, 2024
Aluminum oxide, with the chemical formula Al2O3, is an amphoteric oxide commonly referred to as alumina.
- Aluminium Oxide: Properties and Uses Sep 22, 2020
Aluminum oxide is a common, naturally occurring compound that’s employed in various industries, most particularly in the production of aluminum.
- The applications of Aluminum oxide Sep 24, 2019
Aluminium oxide (IUPAC name) or aluminum oxide (American English) is also known as Alumina. It is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, an
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringNitrogen trifluoride (NF3) is a colorless, nonflammable gas with routine usage in the microelectronics industry.....
Nov 28,2023Inorganic chemistryAluminum oxide
1344-28-1You may like
- An oxide of rare earth metal europium: Europium oxide
Apr 25, 2024
- Tungsten trioxide - Uses, Toxicity, Preparation etc.
Dec 20, 2021
- What is Di-tert-butyl peroxide?
Jul 9, 2021
- Aluminum oxide
-

- 2025-12-12
- CAS:1344-28-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Aluminum oxide
-

- $0.00 / 1KG
- 2025-12-12
- CAS:1344-28-1
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Aluminum oxide
-

- $1.00 / 25KG
- 2025-12-11
- CAS:1344-28-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt