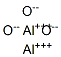The structure of Aluminum oxide
May 24,2024
Description
Aluminum oxide, with the chemical formula Al2O3, is an amphoteric oxide commonly referred to as alumina. Corundum (α-aluminum oxide), emery, sapphire, amethyst, and topaz, as well as many other names, reflect its widespread occurrence in nature and industry. Corundum is the most common naturally occurring crystalline form of aluminum oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colors to trace impurities. Rubies are given their characteristic deep red color and their laser qualities by traces of chromium. Sapphires come in different colors due to other impurities like iron and titanium.
Chemical property
Aluminum oxide does not dissolve in water and has a very high melting point of 2,000 ℃ or about 3,600 F. Its boiling point is an extremely high 5,400 F. The chemical formula combines two aluminum atoms into three oxygen atoms, expressed as Al2O3. It is an electrical resistor, unlike its cousin, aluminum. The resistance level changes with the purity of the material. Aluminum oxide does not react readily with most materials but is highly reactive to chlorine trifluoride and ethylene oxide. Mixing aluminum oxide with either of these chemicals causes a fire.

The crystal structure of αAl2O3 has hexagonal symmetry with the space group R3̅c (no. 167). The structure consists of a hexagonal close-packed array of oxygen atoms along the [001] direction, with the Al atoms occupying two-thirds of the octahedral interstices. Following a full bulk optimization, the predicted equilibrium lattice parameters were a = b = 4.75 Å and c = 12.97 Å, which agrees within 0.21% with the values of a = b = 4.760 Å and c = 12.989 Å reported in the literature[1].
Uses
Alumina injection molding is the manufacturing process that provides specialized custom components for use in different industries, but their main applications are in:
Medical industry: The chemical properties of aluminum oxide, as well as its hardness and bio-inertness, make it a suitable material for various medical applications, including bionic implants, tissue reinforcement, prostheses, hip replacement bearings, etc.
Protective equipment: The lightweight qualities and the strength of aluminum oxide make it a great choice for enhancing body and vehicle armor and creating synthetic-sapphire bulletproof ballistics and windows.
Electrical industry: The high boiling and melting points of aluminum oxide make this compound a great choice for manufacturing high-temperature furnace insulation and electrical insulators. Alumina is also widely used in the microchip industry.
Gem industry: Aluminium oxide is used to form sapphires and rubies. In its crystalline form or corundum, alumina is the base element for creating these two precious
Industrial application: Because aluminum oxide is chemically inert, it is the perfect filler for bricks, plastics, and heavy clayware. It is also often used as the abrasive component of sandpaper and an economical replacement for industrial diamonds.
Safety
Aluminum oxide is not classified as a human carcinogen, but workers chronically exposed to aluminum-containing dust or particles have developed severe pulmonary reactions, including fibrosis, emphysema, and pneumothorax. Inhalation effects of short-term exposure may cause eye and upper respiratory tract irritation. Long-term inhalational effects of long-term exposure may affect the central nervous system.
References
[1] Brian Ramogayana. “Density Functional Theory Study of Ethylene Carbonate Adsorption on the (0001) Surface of Aluminum Oxide α-Al2O3.” ACS Omega 6 44 (2021): 29577–29587.
- Related articles
- Related Qustion
- Aluminium Oxide: Properties and Uses Nov 28, 2023
Aluminum oxide (Al2O3), abundant in Earth's crust, is a compound vital in industries, known for hardness, high-temp resistance, and diverse applications.
- Aluminium Oxide: Properties and Uses Sep 22, 2020
Aluminum oxide is a common, naturally occurring compound that’s employed in various industries, most particularly in the production of aluminum.
- The applications of Aluminum oxide Sep 24, 2019
Aluminium oxide (IUPAC name) or aluminum oxide (American English) is also known as Alumina. It is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, an
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering3,3',4,4'-Biphenyltetracarboxylic dianhydride (BPDA) is a raw material for the synthesis of polyimides.....
May 24,2024APIAluminum oxide
1344-28-1You may like
- Aluminum oxide
-

- 2025-12-03
- CAS:1344-28-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Aluminum oxide
-

- $980.00/ kg
- 2025-12-03
- CAS:1344-28-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Aluminum oxide
-

- $0.00 / 1KG
- 2025-12-03
- CAS:1344-28-1
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month