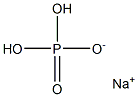
The pharmacokinetics of sodium dihydrogen phosphate
May 5,2022
Introduction
Sodium dihydrogen phosphate, also known as acidic sodium phosphate, with molecular formula of NaH2PO4, is an inorganic acid salt. Soluble in water, almost insoluble in ethanol. Sodium dihydrogen phosphate is the raw material for manufacturing sodium hexametaphosphate and sodium pyrophosphate. It is mainly used in tanning and boiler water treatment, as quality improver and baking powder, as buffer and baking powder raw materials in food industry and fermentation industry, as well as feed additives, detergents and dyeing aids[1].

Picture 1 The physical map of sodium dihydrogen phosphate
Toxicology
Phosphorus in human body exists in organic and inorganic forms. The blood phosphorus measured clinically is the inorganic phosphorus in blood, most of which is free phosphorus, and only 12% is bound to plasma protein. The blood phosphorus concentration of normal adults is 0.87 ~ 1.45mmol/l and that of children is 1.45 ~ 1.78mmol/l. Some reasons lead to the reduction of phosphorus intake or the increase of phosphorus demand, which can cause hypophosphatemia and corresponding clinical manifestations. At this time, phosphorus must be supplemented. There is a close relationship between blood phosphorus and blood calcium concentration. Normally, the product of the two is maintained in a certain range. When the blood calcium concentration increases, phosphate can reduce the blood calcium concentration.
Pharmacokinetics
The oral absorption rate of phosphorus is about 70%, and the absorption site is mainly in the jejunum. Vitamin D can increase the absorption of phosphorus. When eating a large amount of calcium or aluminum at the same time, it will affect the absorption of phosphorus due to the formation of insoluble salt. 90% of phosphorus is excreted by kidney and 10% by feces.
Indication
(1) For the prevention and treatment of hypophosphatemia. It is also used as a phosphorus additive in total intravenous hypernutrition therapy to prevent hypophosphatemia.
(2) As an auxiliary drug for urinary tract infection, this drug can acidify the urine, enhance the antibacterial activity of urotropine amygdala and Urotropine hippurate, and eliminate the smell and turbidity of ammonia containing urine during urinary tract infection.
(3) Prevention of calcium containing kidney stones. This medicine can acidify the urine, increase the solubility of calcium and prevent the deposition of calcium in the urine, so as to prevent the recurrence of calcium containing kidney stones.
(4) Treatment of hypercalcemia. In recent years, this drug is not commonly used to treat hypercalcemia, but other safer and more effective methods are used.
Usage and dosage
When taking this medicine, take it immediately after meals or at the same time with meals to reduce gastrointestinal reactions. It should be completely dissolved in water before taking this medicine.
(1) Hypophosphatemia was treated orally with sodium phosphate solution equivalent to 250mg (8mmol) of phosphorus, 4 times a day; When treating vitamin D rickets, the dosage can be added to 500mg (16mmol) phosphorus each time. In order to prevent ectopic calcification, phosphorus supplement should be stopped when serum phosphorus is greater than 1.5mmol/l (4.5mg / dl). In order to alleviate gastrointestinal symptoms, the solution can be diluted in half a cup of water and taken after meals.
(2) Acidizing urine and preventing recurrence of urinary calculi generally use sodium dihydrogen phosphate and potassium phosphate mixture, with a ratio of about 2:1. Dilute 1g phosphate in a glass of water, take it after meals and before going to bed, 4 times a day. If the above dosage is applied and the urine volume does not reach satisfactory acidification, 1g phosphate can be used every 2 hours and no more than 8g phosphate can be used for 24 hours.
Adverse reaction
(1) Nausea, vomiting, abdominal pain, increased stool frequency or diarrhea may occur during oral administration.
(2) Hypernatremia presents with thirst, rapid heart rate, decreased urine output, headache, dizziness and mental changes.
(3) Hyperkalemia occurs arrhythmia, numbness or tingling of mouth and lip, weakness of limbs and so on.
(4) Hyperphosphatemia and induced hypocalcemia: numbness of hands and feet, tetany, muscle spasm, dyspnea and other phenomena.
(5) retention of water and sodium: edema and weight gain.
Contraindication
Hyperphosphatemia and kidney stones refer to the stones containing magnesium ammonium phosphate caused by infection, serious damage to renal function, and the clearance rate of endogenous creatinine is less than 30% of the normal.
Matters needing attention
(1) Hyperphosphatemia may occur, such as hypoparathyroidism and chronic kidney disease.
(2) Hypocalcemia may occur, such as hypoparathyroidism, osteomalacia, acute pancreatitis and chronic kidney disease.
(3) Edema disease: such as congestive heart failure, acute pulmonary edema, severe liver disease, hypertension, hypernatremia, renal function damage, pregnancy induced hypertension syndrome.
(4) Renal function, blood phosphorus, calcium, sodium and potassium ions were examined according to clinical needs.
Interaction
(1) Taking calcium salt, aluminum hydroxide or magnesium oxide at the same time can reduce the absorption of phosphorus.
(2) combined with adrenal cortex hormone, especially corticosteroids, adrenocorticotropic hormone and androgen, can increase water and sodium retention.
(3) Vitamin D can increase the absorption of oral phosphorus, which is prone to hyperphosphatemia.
Danger
Health hazard: Micro poison. Irritating to eyes and skin. Heat decomposition releases phosphorus oxide and sodium oxide smoke.
Environmental hazard: it is harmful to the environment and can pollute the water body.
Explosion hazard: This product is non combustible and irritating.
First aid measures
Skin contact: take off contaminated clothes and rinse with a large amount of flowing water.
Eye contact: lift the eyelids and rinse with flowing water or normal saline. See a doctor.
Inhalation: quickly leave the site to a place with fresh air. Keep respiratory tract unobstructed. If breathing is difficult, give oxygen. If breathing stops, perform artificial respiration immediately. See a doctor.
Ingestion: drink enough warm water to induce vomiting. See a doctor.
Fire fighting measures
Hazard characteristics: it cannot burn by itself. In case of high heat, it decomposes and releases highly toxic flue gas.
Harmful combustion products: phosphorus oxide and phosphine.
Fire extinguishing method: Firefighters must wear full body fire and poison proof clothes and extinguish the fire in the upwind direction. When extinguishing the fire, move the container from the fire site to an open place as far as possible. Then select appropriate extinguishing agent to extinguish the fire according to the cause of fire.
Operational disposal
Precautions for operation: closed operation, local exhaust. Prevent dust from being released into the air of the workshop. Operators must receive special training and strictly abide by the operating procedures. It is recommended that operators wear self-priming filter dust masks, chemical safety glasses, anti poison penetration work clothes and rubber gloves. Avoid dust generation. Avoid contact with acids. Equipped with leakage emergency treatment equipment. Empty containers may leave harmful substances.
Storage precautions: store in a cool and ventilated warehouse. Keep away from kindling and heat sources. Protect from direct sunlight. Package sealing. It should be stored separately from acids and should not be mixed. The storage area shall be equipped with appropriate materials to contain leakage.
Reference
1 Parsons, Roger, and F_G R. Zobel. "The interphase between mercury and aqueous sodium dihydrogen phosphate." Journal of Electroanalytical Chemistry (1959) 9.5-6 (1965): 333-348.
- Related articles
- Related Qustion
- Sodium Dihydrogenphosphate: An Essential Chemical in Various Applications Nov 29, 2024
Sodium dihydrogenphosphate could be Used to prepare phosphorylated polysaccharide.
N. N-dimethyl ethanolamine is an organic compound with chemical formula C4H11NO. It is mainly used as resin raw material and also as raw material for medicine, dyes and paint solvents.....
May 5,2022APISulfanilamide are generally white crystalline particles or powder; Odorless, bitter at the beginning and slightly sweet after taste....
May 5,2022APISodium phosphate monobasic
7558-80-7You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025
Sodium phosphate monobasic manufacturers
- Sodium dihydrogen orthoporthohosphate
-

- 2025-12-23
- CAS:7558-80-7
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sodium phosphate monobasic
-

- $0.00 / 25KG
- 2025-12-23
- CAS:7558-80-7
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Sodium phosphate monobasic
-

- $100.00 / 50kg
- 2025-12-16
- CAS:7558-80-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton