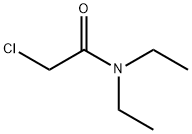
The uses of 2-Chloro-N,N-diethylacetamide in scientific research work
Nov 30,2023
Description
2-Chloro-N,N-diethylacetamide, also known as N, N-diethylchloroacetamide (DECA), belongs to diethyl tertiary amides[1]. It could be used in the preparation of calixarene amides and synthesise 5-bromo-25,26,27,28-tetrakis[(N,N- diethylamino carbonyl)- methoxy] calix arene
Synthesis
Add 425Kg of dichloromethane to the reaction kettle, and then add 90.5Kg of diethylamine, and then add 70.2Kg of chloroacetyl chloride to the reaction kettle under the temperature control condition of 5±5°C, and keep it at 5±5°C after dropping. Moreover, stir for 190 minutes; then add 130.3Kg of water into the reaction kettle, stir for 35 minutes, stand for layering, take the organic layer, add 63.3Kg of water and 23.7Kg of sodium chloride, stir for 35 minutes, stand for layering, and take the organic layer. 50±3 normal pressure recovery dichloromethane to the condenser, then 50±3 reduced pressure to recover the dichloromethane until the condenser does not come out; then keep the temperature and vacuum constant, continue to recover for 5 hours, put N, N-diethyl chloroacetamide is obtained from the raw material with a purity of 98.4% and a yield of nearly 100%.
Research
Buyle et al. use N, N-Diethylchloroacetamide to synthesize ynamine amide and ynamine ester via its β-chloro-β-chloroacyl-β-chloroenamine by chlorine elimination with lithium amalgam[2].
Solovieva et al. studied the interaction of p-tert-butylthiacalix arene with N, N-diethylchloroacetamide in the presence of alkali metal carbonates in acetone. The study of the reaction of thiacalix arene with N, N-diethylchloroacetamide showed that the stereochemical result of the reaction depends on the nature of the base used (template effect), as in the case of the reactions with ethyl bromoacetate and α-bromoacetophenone[3].

N, N-diethylchloroacetamide bearing both a leaving group and electron-withdrawing group. It could be used to synthesize new spiro[cyclopropane-1,4′-pyrazol-3-one] derivative, 5,6-Diaza-N, N-diethyl-4-methyl-7-oxo-2,2,6-triphenylspiro[2.4]hept-4-ene-1-carboxamide, by the react with 4-arylidene-3H-pyrazol-3-one. The synthesis diagram is shown above. The synthesized compounds were active against Candida albicans with MIC ≤ 25 μg/mL in vitro[4].
References
[1] R. Sudarshan, V. V. Chalapathi, G. Ramana Rao. “Vibrational spectra and normal coordinate analysis of some diethyl tertiary amides.” Journal of Raman Spectroscopy 21 7 (1990): 407–415.
[2] S. E. Solovieva. “Synthesis, structure, and complexation properties of tetraamide derivatives of thiacalix[4]arene in different conformations.” Russian Chemical Bulletin 54 9 (2005): 2104–2112.
[3] R. Buyle, H.G. Viehe. “α-Chloro-enamines—III.” Tetrahedron 25 16 (1969): Pages 3447-3451.
[4] Hiroshi Maruoka. “Synthesis and antifungal activity of spiro[cyclopropane-1,4′-pyrazol-3-one] derivatives.” Journal of Heterocyclic Chemistry 45 6 (2009): 1883–1887.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
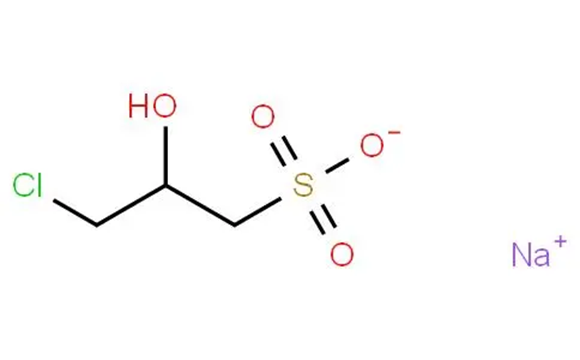
Nov 4,2025Biochemical EngineeringSodium 3-chloro-2-hydroxypropanesulfonate can be used to prepare a hydroxysulfobetaine and surface-derivatised microcrystalline cellulose (MCC) particles.....
Nov 30,2023API2-Chloro-N,N-diethylacetamide
2315-36-8You may like
2-Chloro-N,N-diethylacetamide manufacturers
- N,N-Diethylchloroacetamide
-

- $10.00 / 1kg
- 2025-12-03
- CAS:2315-36-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- N,N-Diethylchloroacetamide
-

- $1.10 / 1g
- 2025-11-18
- CAS:2315-36-8
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min
- Amylin, amide, rat acetate(124447-81-0,free base)
-
- $178.00 / 1mg
- 2025-10-09
- CAS:
- Min. Order:
- Purity: 98.93%
- Supply Ability: 10g