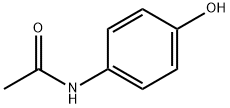
What is Acetaminophen used for?
Oct 12,2021
Acetaminophen can be found as the active ingredient in more than 100 over-the-counter products and a number of prescription drugs, alone or in combination with other drugs. The pharmacology and toxicology of this drug have been extensively studied and reviewed. The first clinical use of acetaminophen dates back to 1893 by von Mering (and subsequently by Hinsberg and Treupel, 1894) as an effective antipyretic with comparable pharmacological effects to antipyrine and phenacetin. However, after a hiatus of almost half a century, acetaminophen was rediscovered as the major metabolite of phenacetin and acetanilide in man and was marketed in the United States as a combination with aspirin and caffeine in 1950.
In the 1960s and 1970s, concerns about gastrointestinal adverse effects of aspirin and methemoglobinemia of acetanilide only led to increased popularity of acetaminophen as a generally safe antipyretic analgesic. Hepatotoxicity of acetaminophen began to be reported in the late 1960s and has been a topic of intense scientific evaluationto this day. The impact of acetaminophen-induced liver toxicity, accidental or otherwise, will be taken up in later sections.

Uses
Acetaminophen is a nonnarcotic analgesic and antipyretic drug. It is used to relieve pain of moderate intensity, such as usually occurs in headache and in many muscle, joint, and peripheral nerve disorders. Headaches are one of the most common indications for the use of acetaminophen. Acetaminophen is used to treat acute tension headaches and mild to moderate migraine, especially in combination with caffeine and aspirin.
Acetaminophen is indicated in chronic pain associated with rheumatoid arthritis, back or hip pain, osteoarthritis, dental pain, or acute pain due to soft-tissue injury. Acetaminophen is a suitable substitute for aspirin for its analgesic or antipyretic uses in cases where aspirin is contraindicated (gastric bleeding) or when the prolongation of bleeding time caused by aspirin would be a disadvantage.
Acetaminophen has been used in studies of pain relief following obstetric and gynecological procedures, including Caesarean section, hysterectomy, tubal ligation, primary dysmenorrhea, and termination of pregnancy. Acetaminophen is also used to manage chronic pain of cancer, postpartum pain, and postoperative pain after minor surgery.
In a double-blind crossover study, the analgesic oral butorphanol, and acetaminophen in combination, showed additive analgesic effects against moderate to severe pain due to metastatic carcinoma over that of the individual drug. Acetaminophen is also widely used as an antipyretic drug to reduce fever.
Environmental Fate
Although a major part of the ingested dose of acetaminophen is detoxified, a very small proportion is metabolized via the cytochrome P450-mixed function oxidase pathway to a highly reactive n-acetyl-p-benzoquinoneimine (NAPQI). The toxic intermediate NAPQI is normally detoxified by endogenous glutathione to cysteine and mercapturic acid conjugates and excreted in the urine. Recent studies have shown that hepatic P450s, CYP2E1, and to a lesser extent CYP1A2 are responsible for conversion of acetaminophen to NAPQI. In acetaminophen overdose, the amount of NAPQI increases and depletes endogenous glutathione stores. Time course studies have shown that covalent binding of reactive NAPQI and subsequent toxicity occur only after cellular glutathione stores are reduced by 70% or more of normal. Mitochondrial dysfunction and damage can be seen as early as 15 min after a toxic dose in mice, suggesting that this may be a critical to cellular necrosis. The NAPQI is then thought to covalently bind to critical cellular macromolecules in hepatocytes and cause cell death.
Recent proteomic studies have identified at least 20 known proteins that are covalently modified by the reactive acetaminophen metabolite. The resulting acetaminophen-cysteine (APAP-CYS) protein adducts can be quantified via a highpressure liquid chromatography coupled with electrochemical detection (HPLC-EC). Hepatic necrosis and inflammation develop as a consequence of hepatocellular death, which results in development of clinical and laboratory findings consistent with liver failure. A similar mechanism is postulated for the renal damage that occurs in some patients following acetaminophen toxicity.
- Related articles
- Related Qustion
- Acetaminophen:Synthesis,Uses,Side effects,safe dosage Jul 25, 2024
Acetaminophen is most commonly used to treat minor aches and pains, including headache, backache, minor pain of arthritis, toothache, muscular aches, premenstrual and menstrual cramps.
- Usage and Side effects of Tylenol Mar 19, 2024
The active ingredient in Tylenol is paracetamol, a widely used over-the-counter analgesic (pain reliever) and antipyretic (fever reducer).
- The mechanism of action of Acetaminophen and its toxicity Mar 7, 2024
Acetaminophen inhibits the synthesis of prostaglandins and is commonly used to treat fever caused by the common cold or flu and to relieve mild to moderate pain symptoms such as headaches and joint pain.
Acetamide is a member of the class of acetamides that results from the formal condensation of acetic acid with ammonia. It is a monocarboxylic acid amide, a N-acylammonia and a member of acetamides. It is a tautomer of an acetimidic acid.....
Oct 12,2021APIDMM has limited use because of its toxicity but can be used to calibrate research equipment, as in its application as a standard reference material for 199Hg NMR measurements.....
Oct 13,2021Organometallic compoundsAcetaminophen
103-90-2You may like
- Acetaminophen
-

- $1.00 / 1kg
- 2025-12-06
- CAS:103-90-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 tons
- Acetaminophen
-

- $0.00 / 1KG
- 2025-12-06
- CAS:103-90-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1 ton/year
- Acetaminophen
-

- $0.00 / 1kg
- 2025-12-06
- CAS:103-90-2
- Min. Order: 1kg
- Purity: 99%pure
- Supply Ability: 20 tons