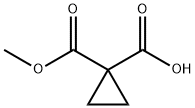
1,1-Cyclopropanedicarboxylic acid monomethyl ester synthesis
- Product Name:1,1-Cyclopropanedicarboxylic acid monomethyl ester
- CAS Number:113020-21-6
- Molecular formula:C6H8O4
- Molecular Weight:144.13
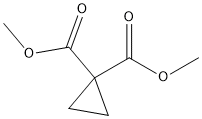
6914-71-2

113020-21-6
1. KOH (42 mg, 0.75 mmol) was added to a methanolic solution of dimethyl cyclopropane-1,1-dicarboxylate (100 mg, 0.63 mmol). 2. The reaction mixture was stirred at room temperature for 4 hours. 3. After completion of the reaction, the solvent was removed by concentration under reduced pressure. 4. The residue was diluted with water and acidified with concentrated hydrochloric acid to pH<2. 5. The acidified aqueous phase was extracted with dichloromethane (3 x 10 mL). 6. The organic phases are combined and dried over anhydrous sodium sulfate. 7. The desiccant was removed by filtration and the filtrate was concentrated under reduced pressure to give cyclopropane-1,1-dicarboxylic acid monomethyl ester (65 mg, 71% yield) as a white solid.

67-56-1
790 suppliers
$7.29/5ml-f
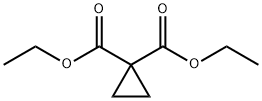
1559-02-0
323 suppliers
$5.00/5g

113020-21-6
183 suppliers
$5.00/1g
Yield:113020-21-6 79%
Reaction Conditions:
with potassium hydroxide at 0 - 20; for 2 h;
Steps:
171 Intermediate 171a: l-(methoxycarbonyl)cyclopropanecarboxylic acid
To 1,1- diethyl cyclopropane- 1,1 -dicarboxylate (15.0 g, 80,56 mmol, 1.00 equiv) in methanol (90 mL) at 0 °C was added batch- wise potassium hydroxide (6.3 g, 112.28 mmol, 1.40 equiv) and the resulting solution allowed to warm to RT and stirred for 2 b. The mixture was concentrated under vacuum., diluted with 100 mL of water and then washed with 1 x 50 mL of ethyl acetate. The pH value of the aqueous solution was adjusted to 3-4 with cone. HCl, extracted with 3 x 50 mL of ethyl acetate, the organic layers combined and then washed with brine. The organic layer was dried over anhydrous, sodium sulfate and concentrated to afford 9.2 g (79%) of intermediate 171a as a colorless liquid
References:
ARDELYX, INC.;LEWIS, Jason, G.;REICH, Nicholas;CHEN, Tao;JACOBS, Jeffrey W.;CHARMOT, Dominique;NAVRE, Marc;FINN, Patricia;CARRERAS, Christopher;SPENCER, Andrew WO2013/96771, 2013, A1 Location in patent:Page/Page column 229

6914-71-2
210 suppliers
$35.00/25 g

113020-21-6
183 suppliers
$5.00/1g

6914-71-2
210 suppliers
$35.00/25 g
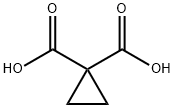
598-10-7
360 suppliers
$7.00/1g

113020-21-6
183 suppliers
$5.00/1g