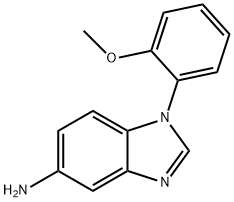
1-(2-methoxyphenyl)-1H-benzo[d]imidazol-5-amine synthesis
- Product Name:1-(2-methoxyphenyl)-1H-benzo[d]imidazol-5-amine
- CAS Number:380641-63-4
- Molecular formula:C14H13N3O
- Molecular Weight:239.27

64-18-6
1112 suppliers
$22.12/250ML
14038-08-5
11 suppliers
$28.60/1g
![1-(2-methoxyphenyl)-1H-benzo[d]imidazol-5-amine](/CAS/20200611/GIF/380641-63-4.gif)
380641-63-4
19 suppliers
$240.00/100MG
Yield:380641-63-4 100%
Reaction Conditions:
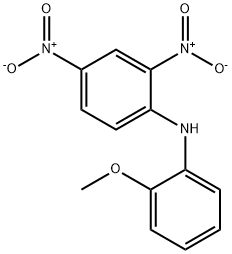
Stage #1: N-(2,4-Dinitrophenyl)-2-methoxy-anilinewith hydrogen;palladium 10% on activated carbon in ethyl acetate; for 91 h;
Stage #2: formic acidwith hydrogenchloride in water at 110; for 2.75 h;Inert atmosphere;
Stage #3: with sodium hydroxide in water at 20; pH=12;
Steps:
15.b
To a solution of 2,4-dinitro-JV- (2-methoxyphenyl)benzamine (2) (2.72 g, 9.4 mmol) in ethyl acetate (95 mL) is added 10% Pd/C (325 mg). The reaction mixture is stirred under H2 for 91 h and then filtered through Celite (Ethyl acetate rinse). After solvent removal at reduced pressure, the residue is used in the next step without further purification.[0188] The solution of the residue and formic acid (1.5 mL, 38 mmol) in 4 N HCl (32 mL) is heated at 110 °C under argon for 2.75 h. The reaction mixture is cooled to room temperature, brought to pH 12 with NaOH pellets, and extracted with ethyl acetate (150 mL, 100 mL, and 50 mL). The extract is washed (brine) and dried. After solvent removal at reduced pressure, the residue is purified on silica gel (1% to 6% methanol/ethyl acetate) to give 2.3 g (100%) of 3 as a red-brown solid, mp 48-50 °C. IR 3323, 2951, 1510, 1260 cm"1; 1H NMR (CDCI3) δ 2.98 (bs, 2H, NH2, 3.83 (s, 3H, CH3), 6.74 (dd, J= 8.4 Hz, 1.8 Hz, IH, 6- BenzimidH), 7.10-7.20 (m, 4H, 4,7-BenzimidH, 3,5-PhH), 7.40-7.49 (m, 2H, 4,6-PhH), 8.00 ppm(s, IH, 2-BenzimidH). HRMS calcd C14H13N3O [M + H]+ 240.1131, found 240.1133.
References:
WO2010/33643,2010,A2 Location in patent:Page/Page column 59; 60