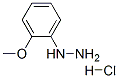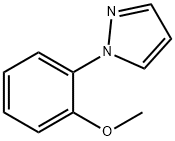
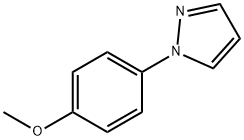
1-(2-Methoxyphenyl)-1H-pyrazole synthesis
- Product Name:1-(2-Methoxyphenyl)-1H-pyrazole
- CAS Number:102908-37-2
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
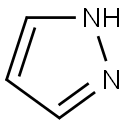
288-13-1
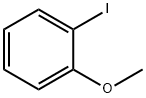
529-28-2

102908-37-2
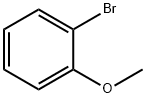
GENERAL METHOD: Complex 2 (0.05 mmol) was added to a 5 mL sealed tube containing 2-iodoanisole (0.5 mmol), 1H-pyrazole (0.75 mmol), sodium hydroxide (1 mmol), and dimethyl sulfoxide (1 mL). The reaction mixture was stirred at 100 °C for 12 hours. Upon completion of the reaction, it was cooled to room temperature and the reaction was quenched with 10 mL of water, followed by extraction with ethyl acetate (3 x 20 mL). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by fast column chromatography on silica gel with petroleum ether/ethyl acetate (10:1 to 5:1) as eluent to give 1-(2-methoxyphenyl)-1H-pyrazole. All N-arylpyrazole products were known compounds and their structures were confirmed by 1H NMR and GC-MS.
Yield:102908-37-2 179.4 mg
Reaction Conditions:
in ethanol; for 2 h;Reflux;
Steps:
3.a 1-(2-Methoxyphenyl)-1H-pyrazole
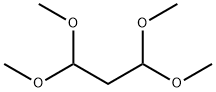
200 mg of 2-methoxyphenylhydrazine hydrochloride was dissolved in 5 ml of ethanol and 189 μl of malonaldehyde bisdimethylacetal was added, followed by heating to reflux for 2 hours. To the reaction mixture was added 50 ml of water, followed by neutralizing with a saturated aqueous sodium carbonate solution and extracting with 60 ml of ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate and subsequently the solvent was distilled off under reduced pressure to afford 179.4 mg of the title compound. 1H-NMR (CDCl3); δ (ppm) 3.87 (3H,s), 6.42 (1H, d, J=2.4Hz), 7.02-7.07 (2H, m), 7.27-7.32 (1H, m), 7.68-7.72 (2H, m), 8.01 (1H, d, J=2.4Hz). MS (FAB); m/z 175 (M+H)+
References:
EP2889031,2015,A1 Location in patent:Paragraph 0128

288-13-1
456 suppliers
$10.00/1g
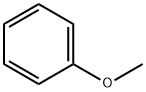
100-66-3
539 suppliers
$10.00/5G

102908-37-2
30 suppliers
inquiry
35715-67-4
51 suppliers
$63.00/1g