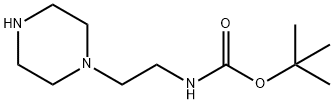
1-(2-N-Boc-Aminoethyl)piperazine synthesis
- Product Name:1-(2-N-Boc-Aminoethyl)piperazine
- CAS Number:140447-78-5
- Molecular formula:C11H23N3O2
- Molecular Weight:229.32
500013-42-3

140447-78-5
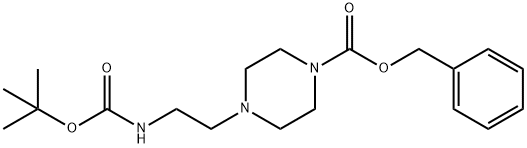
The general procedure for the synthesis of 1-(N-Boc-aminoethyl)piperazine from 1-CBZ-4-(BOC-aminoethyl)piperazine was as follows: hydrogen was vented into a degassed solution of benzyl 4-(2-(tert-butoxycarbonylamino)ethyl)piperazine-1-carboxylate (2.60 g, 7.16 mmol) containing 10% palladium carbon (500 mg) in methanol (50 ml), the reaction lasted for 2 hours. After completion of the reaction, the mixture was filtered through Celite. The filtrate was concentrated under vacuum to give a yellow colloidal product which was identified as tert-butyl 2-(1-piperazinyl)ethylcarbamate (1.60 g, 97% yield).

500013-42-3
9 suppliers
inquiry

140447-78-5
176 suppliers
$9.00/100mg
Yield:140447-78-5 97%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol for 2 h;
Steps:
22.D 22D: tert-Butyl 2-(1-piperazinyl)ethylcarbamate
Hydrogen was passed through a degassed solution of benzyl 4-(2-(tert-butyloxycarbonylamino)ethyl)piperazine-1-carboxylate (2.60 g, 7.16 mmol) in methanol (50 ml) containing 10% palladium on carbon (500 mg) for 2 h. The reaction mixture was filtered through Celite and the filtrate was concentratedin vacuo to give a yellow gum identified as tert-butyl 2-(1-piperazinyl)ethylcarbamate (1.60 g, 97%).
References:
Ferring B.V. EP1449844, 2004, A1 Location in patent:Page 34
![Carbamic acid, N-[2-[4-(phenylmethyl)-1-piperazinyl]ethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1007869-45-5.gif)
1007869-45-5
1 suppliers
inquiry

140447-78-5
176 suppliers
$9.00/100mg

110-85-0
589 suppliers
$5.00/5 g
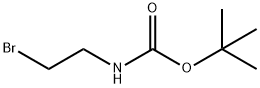
39684-80-5
257 suppliers
$17.30/250mg

140447-78-5
176 suppliers
$9.00/100mg

24424-99-5
868 suppliers
$13.50/25G

140447-78-5
176 suppliers
$9.00/100mg
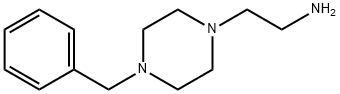
4553-21-3
89 suppliers
$17.67/250mgs:

140447-78-5
176 suppliers
$9.00/100mg